15.7:
Weak Acid Solutions
A weak acid, like hydrocyanic acid, is a Brønsted acid as it donates a proton to the water molecule and produces the hydronium ion. A weak acid dissociates partially in water according to its acid dissociation constant, Ka, which is 4.9 × 10−10 for hydrocyanic acid.
For hydrocyanic acid, the Ka is equal to the concentration of hydronium times the concentration of cyanide ions divided by the concentration of hydrocyanic acid.
The acid dissociation constant, Ka, can be used to determine the hydronium ion concentration in a weak acid solution and consequently, the pH of the solution.
The concentration of hydronium ions and the pH of a 0.15 molar solution of hydrocyanic acid can be calculated using its equilibrium expression and an ICE table.
The concentrations of hydrocyanic acid, hydronium, and cyanide initially and at equilibrium can be expressed in a table that shows the Initial, Change, and Equilibrium concentrations of each of the molecules.
To reach equilibrium, the initial concentration of the reactants decreases as the initial concentration of the products increases according to their molar ratios. This change in the concentration of the reactants and products is denoted by x.
Substituting equilibrium concentrations in the expression for the Ka yields x times x divided by 0.15 minus x.
In many weak acids, x, the amount of dissociation, is likely to be very small compared to the initial concentration of 0.15 molar. 0.15 minus x can be assumed to be approximately 0.15.
When the equation is solved, x equals 8.6 × 10−6 molar.
The approximation, 0.15 minus x equal to 0.15, is valid only if x is less than 5% of 0.15 molar. Here, x is 0.0057% of 0.15 molar and hence this approximation is valid.
Therefore, the concentration of hydronium is 8.6 × 10−6 molar. To determine the pH, take the negative log of the hydronium ion concentration. Solving this shows the pH of the 0.15 M hydrocyanic acid solution is 5.07.
The pH of a solution can be used to determine the Ka of a weak acid.
For example, acetic acid dissociates partially into hydronium ions and acetate ions when dissolved in water. The Ka for acetic acid can be expressed as the hydronium ion concentration times the acetate ion concentration divided by the concentration of acetic acid.
If the pH of a 0.20 molar acetic acid solution is 2.72, its hydronium concentration can be calculated, which is 1.9 × 10−3 molar.
The ICE table can be constructed from the initial and equilibrium concentrations of the acetic acid, hydronium ions, and acetate ions.
Using significant figures, 0.20 minus 1.9 × 10−3 is essentially equal to 0.20. By substituting the equilibrium values into the Ka expression, Ka equals 1.8 × 10−5.
15.7:
Weak Acid Solutions
Few compounds act as strong acids. A far greater number of compounds behave as weak acids and only partially react with water, leaving a large majority of dissolved molecules in their original form and generating a relatively small amount of hydronium ions. Weak acids are commonly encountered in nature, being the substances partly responsible for the tangy taste of citrus fruits, the stinging sensation of insect bites, and the unpleasant smells associated with body odor. A familiar example of a weak acid is acetic acid, the main ingredient in vinegar:
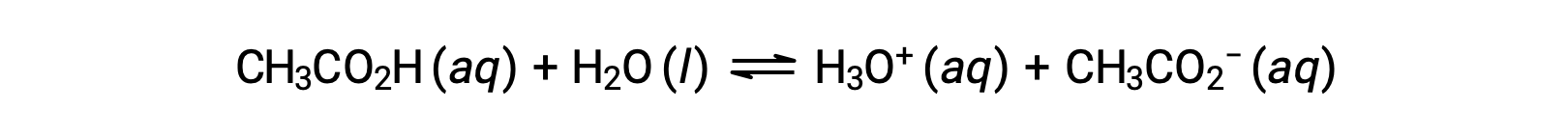
The use of a double-arrow in the equation above denotes the partial reaction aspect of this process. When dissolved in water under typical conditions, only about 1% of acetic acid molecules are present in the ionized form, CH3COO−.
Calculating Hydronium Ion Concentrations and the pH of a Weak Acid Solution
Formic acid, HCO2H, is one irritant that causes the body’s reaction to some ant bites and stings. What is the concentration of hydronium ion and the pH of a 0.534-M solution of formic acid?
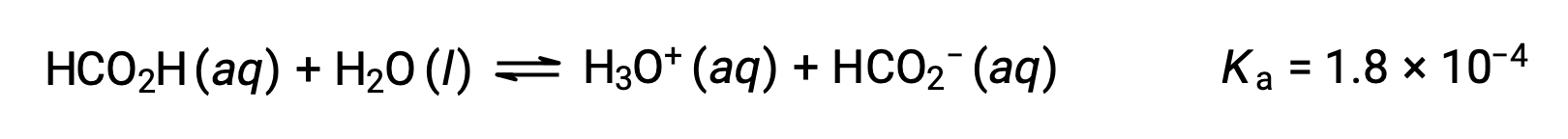
The ICE table for this system is
| HCO2H (aq) | H3O+ (aq) | HCO2− (aq) | |
| Initial Concentration (M) | 0.534 | ~0 | 0 |
| Change (M) | −x | +x | +x |
| Equilibrium Concentration (M) | 0.534 − x | x | x |
Substituting the equilibrium concentration terms into the Ka expression gives
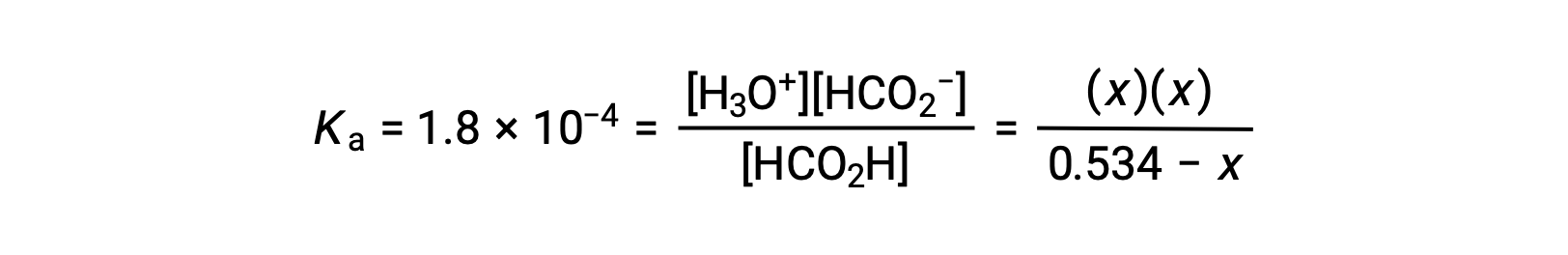
The relatively large initial concentration and small equilibrium constant permits the simplifying assumption that x will be much lesser than 0.534, and so the equation becomes
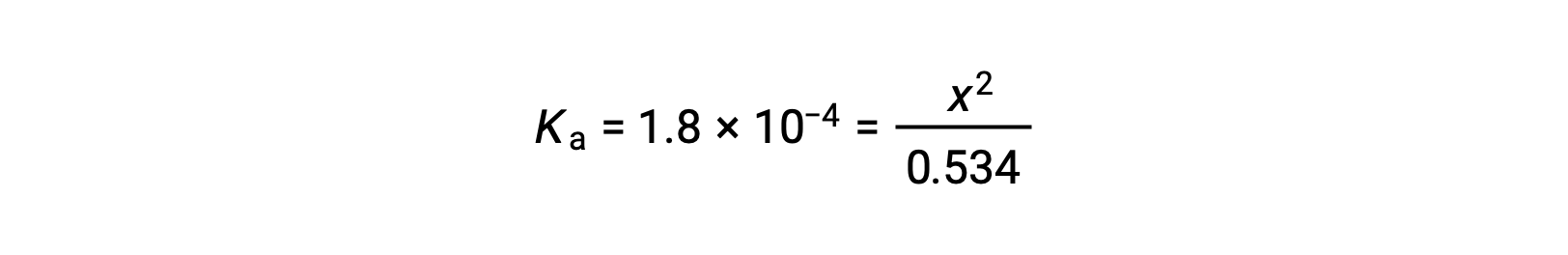
Solving the equation for x yields
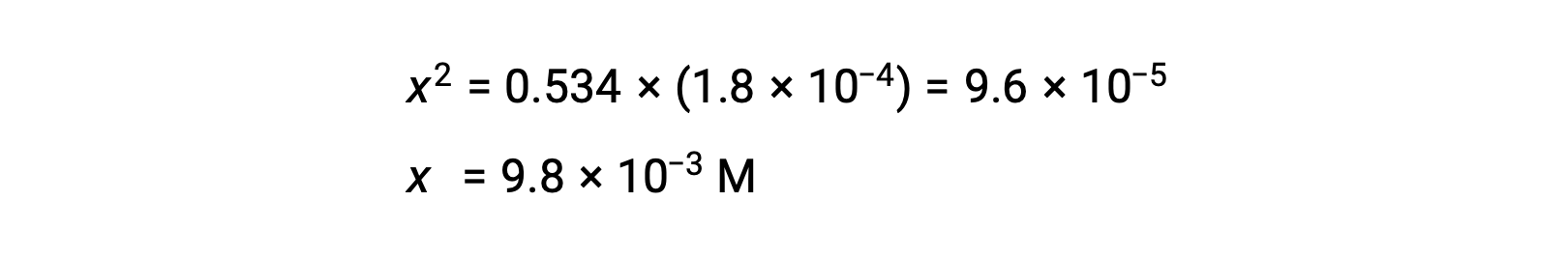
To check the assumption that x is small compared to 0.534, its relative magnitude can be estimated:
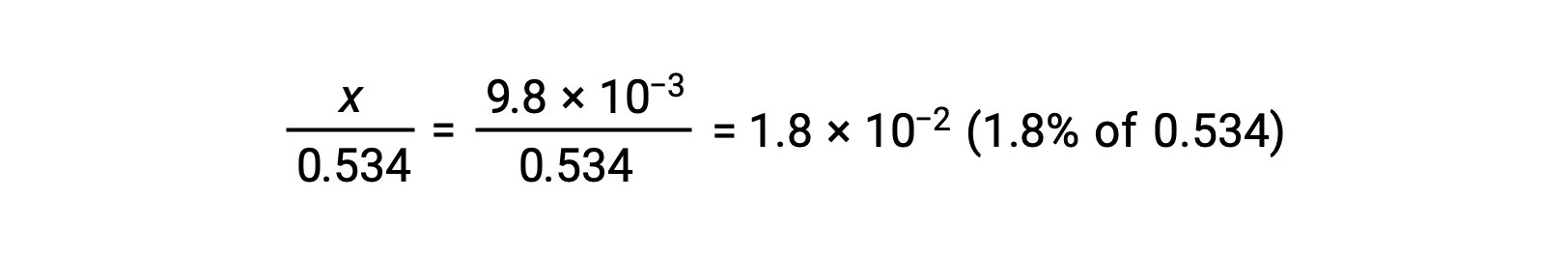
Because x is less than 5% of the initial concentration, the assumption is valid. As defined in the ICE table, x is equal to the equilibrium concentration of hydronium ion:
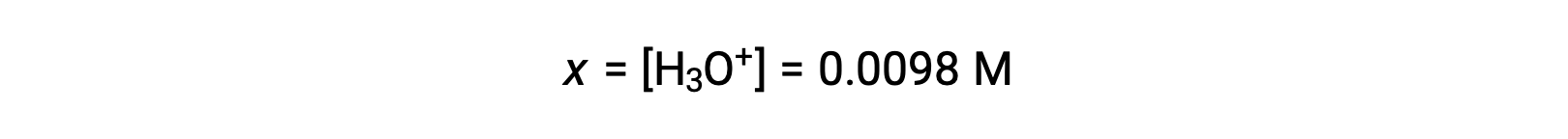
Finally, the pH is calculated to be
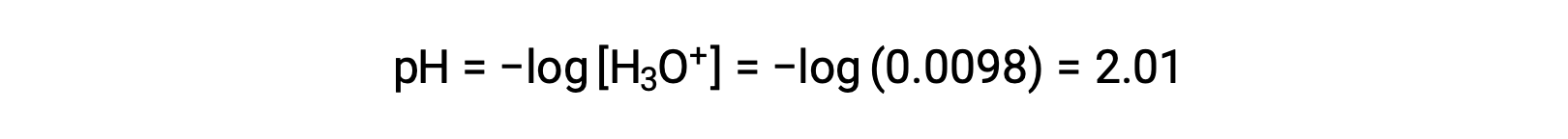
Determination of Ka from pH
The pH of a 0.0516 M solution of nitrous acid, HNO2, is 2.34. What is its Ka?
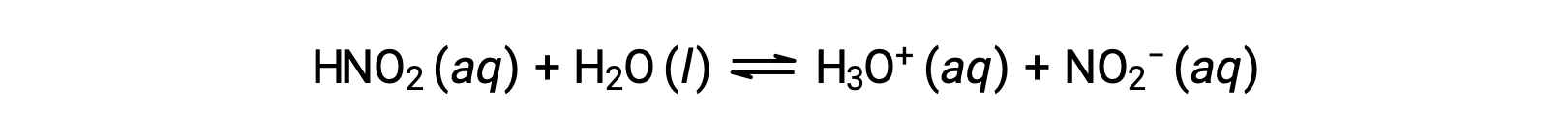
The nitrous acid concentration provided is a formal concentration, one that does not account for any chemical equilibria that may be established in solution. Such concentrations are treated as “initial” values for equilibrium calculations using the ICE table approach. Notice the initial value of hydronium ion is listed as approximately zero because a small concentration of H3O+ is present (1 × 10−7 M) due to the autoionization of water. In many cases, this concentration is much less than that generated by ionization of the acid (or base) in question and may be neglected.
The pH provided is a logarithmic measure of the hydronium ion concentration resulting from the acid ionization of the nitrous acid, and so it represents an “equilibrium” value for the ICE table:
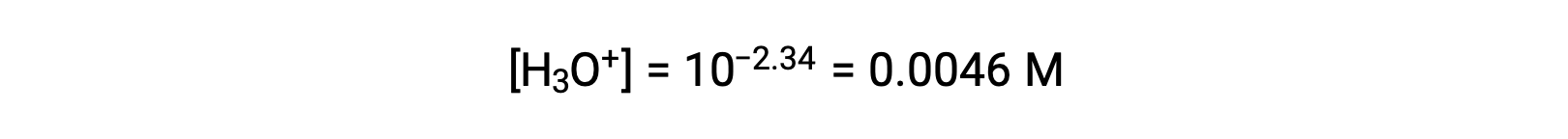
The ICE table for this system is then
| HNO2 (aq) | H3O+ (aq) | NO2− (aq) | |
| Initial Concentration (M) | 0.0516 | ~0 | 0 |
| Change (M) | −0.0046 | +0.0046 | +0.0046 |
| Equilibrium Concentration (M) | 0.0470 | 0.0046 | 0.0046 |
Finally, calculate the value of the equilibrium constant using the data in the table:
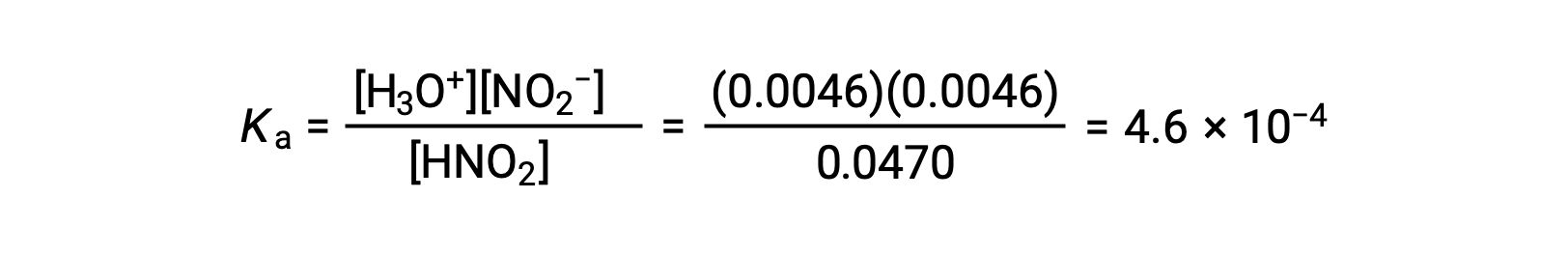
This text is adapted from Openstax, Chemistry 2e, Section 4.2: Classifying Chemical Reactions and Openstax, Chemistry 2e,14.3 Relative Strengths of Acids and Bases.
