15.10:
Ions as Acids and Bases
15.10:
Ions as Acids and Bases
Salts with Acidic Ions
Salts are ionic compounds composed of cations and anions, either of which may be capable of undergoing an acid or base ionization reaction with water. Aqueous salt solutions, therefore, may be acidic, basic, or neutral, depending on the relative acid-base strengths of the salt’s constituent ions. For example, dissolving the ammonium chloride in water results in its dissociation, as described by the equation:
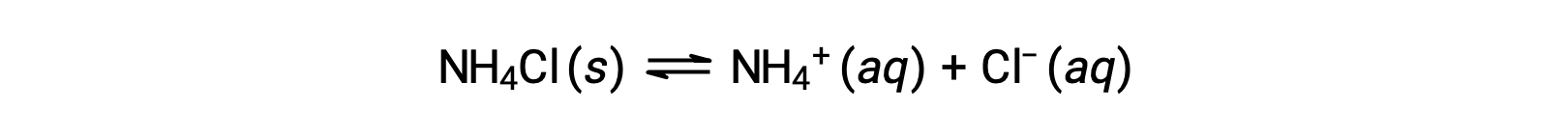
The ammonium ion is the conjugate acid of the base ammonia, NH3; its acid ionization (or acid hydrolysis) reaction is represented by
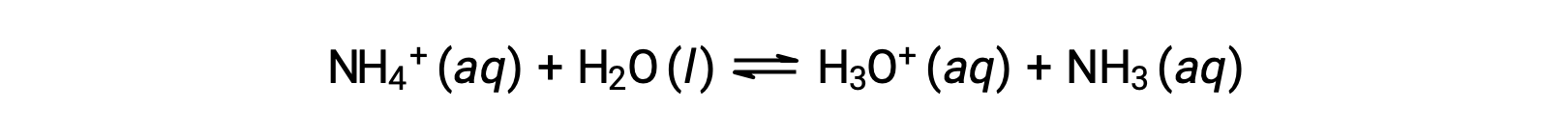
Since ammonia is a weak base, Kb is measurable and Ka > 0 (ammonium ion is a weak acid).
The chloride ion is the conjugate base of hydrochloric acid, and so its base ionization (or base hydrolysis) reaction is represented by
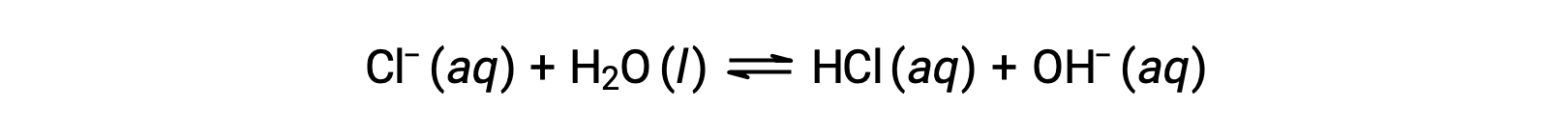
Since HCl is a strong acid, Ka is immeasurably large and Kb ≈ 0 (chloride ions don’t undergo appreciable hydrolysis). Thus, dissolving ammonium chloride in water yields a solution of weak acid cations (NH4+) and inert anions (Cl−), resulting in an acidic solution.
Salts with Basic Ions
As another example, consider dissolving sodium acetate in water:
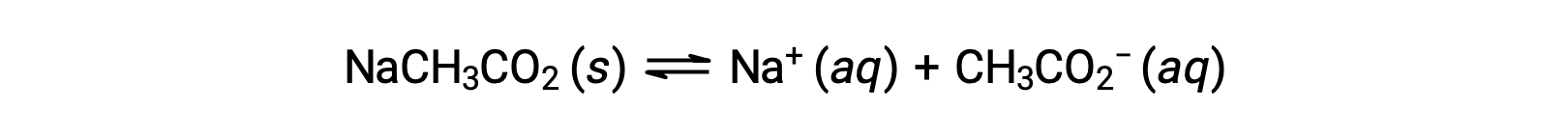
The sodium ion does not undergo appreciable acid or base ionization and has no effect on the solution pH. This may seem obvious from the ion's formula, which indicates no hydrogen or oxygen atoms, but some dissolved metal ions function as weak acids, as addressed later in this section. The acetate ion, CH3CO2−, is the conjugate base of acetic acid, CH3CO2H, and so its base ionization (or base hydrolysis) reaction is represented by
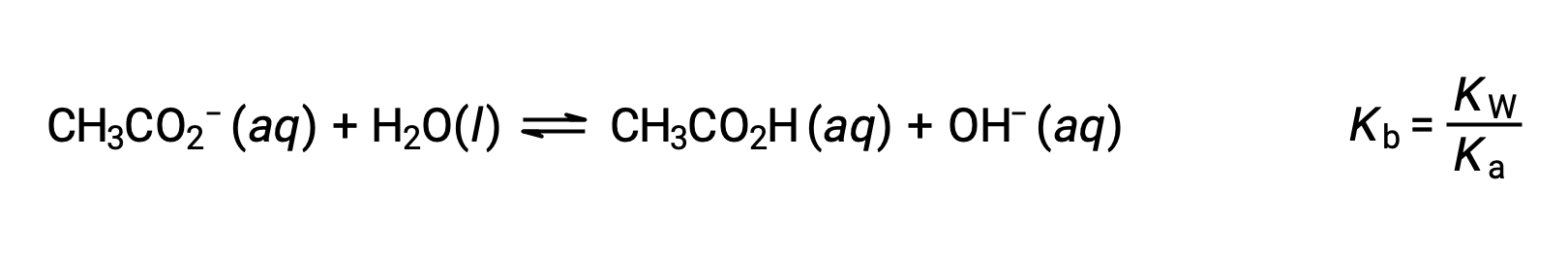
Because acetic acid is a weak acid, its Ka is measurable and Kb > 0 (acetate ion is a weak base). Dissolving sodium acetate in water yields a solution of inert cations (Na+) and weak base anions (CH3CO2−), resulting in a basic solution.
Salts with Acidic and Basic Ions
Some salts are composed of both acidic and basic ions, and so the pH of their solutions will depend on the relative strengths of these two species. For such types of salts, a comparison of the Ka and Kb values allows the prediction of the solution’s acid-base status.
The Ionization of Hydrated Metal Ions
Unlike the group 1 and 2 metal ions of the preceding examples (Na+, Ca2+, etc.), some metal ions function as acids in aqueous solutions. These ions are not just loosely solvated by water molecules when dissolved; instead they are covalently bonded to a fixed number of water molecules to yield a complex ion (see the chapter on coordination chemistry). As an example, the dissolution of aluminum nitrate in water is typically represented as

However, the aluminum(III) ion actually reacts with six water molecules to form a stable complex ion, and so the more explicit representation of the dissolution process is
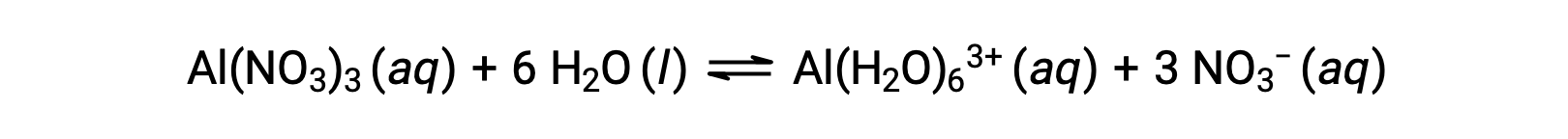
The Al(H2O)63+ ions involve bonds between a central Al atom and the O atoms of the six water molecules. Consequently, the bonded water molecules' O–H bonds are more polar than in nonbonded water molecules, making the bonded molecules more prone to the donation of a hydrogen ion:
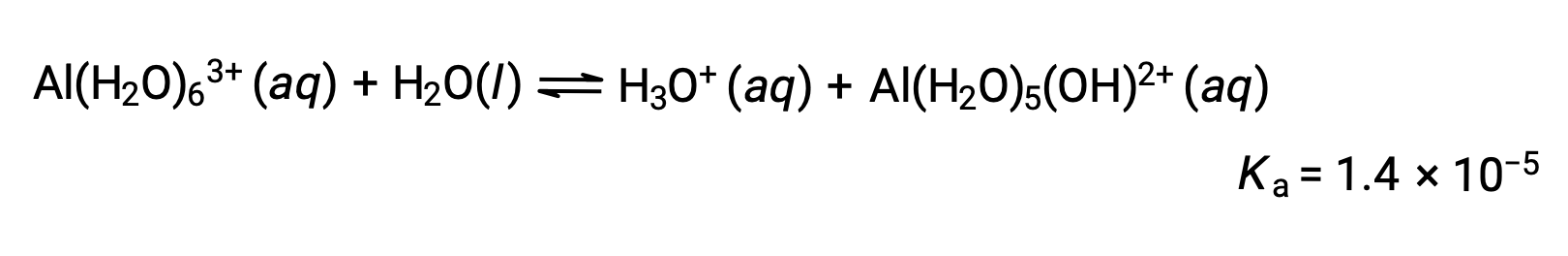
The conjugate base produced by this process contains five other bonded water molecules capable of acting as acids, and so the sequential or step-wise transfer of protons is possible as depicted in few equations below:
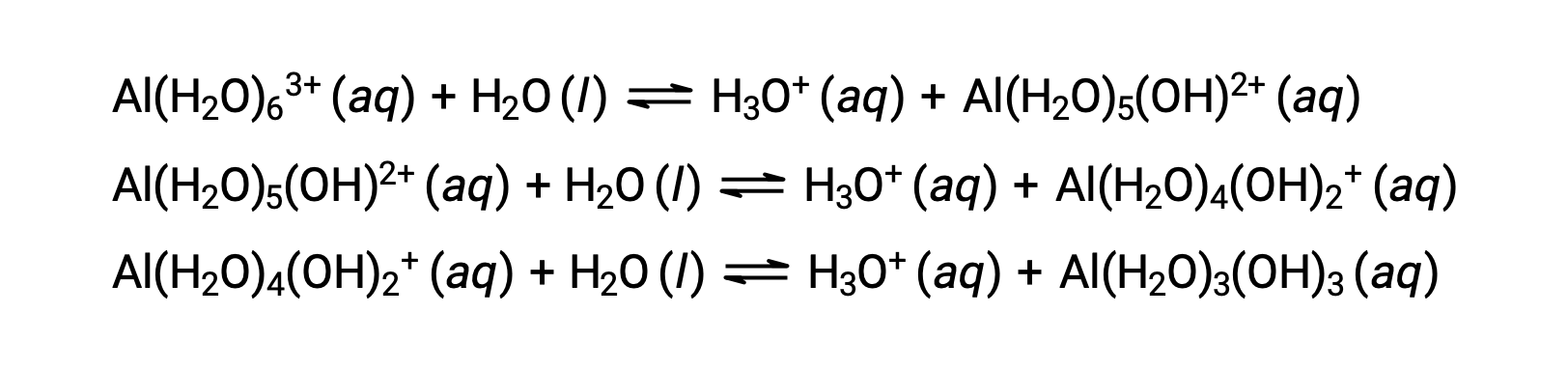
Aside from the alkali metals (group 1) and some alkaline earth metals (group 2), most other metal ions will undergo acid ionization to some extent when dissolved in water. The acid strength of these complex ions typically increases with increasing charge and decreasing size of the metal ions. The first-step acid ionization equations for a few other acidic metal ions are shown below:
| First-step Ionization Equations | pKa |
| Fe(H2O)63+ (aq) + H2O (l) ⇌ H3O+ (aq) + Fe(H2O)5(OH)2+ (aq) | 2.74 |
| Cu(H2O)62+ (aq) + H2O (l) ⇌ H3O+ (aq) + Cu(H2O)5(OH)+ (aq) | ~6.3 |
| Zn(H2O)42+ (aq) + H2O (l) ⇌ H3O+ (aq) + Zn(H2O)3(OH)+ (aq) | 9.6 |
This text is adapted from Openstax, Chemistry 2e, Section 14.4: Hydrolysis of Salts.
