15.11:
Determining the pH of Salt Solutions
Salt solutions can be acidic, basic, or neutral. The individual ions can be examined to determine if the pH of its solution will be below, above, or equal to seven.
Salts that contain pH-neutral cations and anions form neutral solutions. These salts are composed of the counterions of a strong acid and a strong base. A pure aqueous solution of potassium nitrate will have a pH of 7, as neither potassium nor nitrate has acidic or basic properties.
Salts that contain a cation that is the counterion of a weak base and an anion that is the counterion of a strong acid will have a pH less than 7. In a solution of ammonium bromide, the bromide ions are pH neutral, whereas the ammonium ions are acidic and donate protons to the water molecules.
Salts that contain a cation that is the counterion of a strong base and an anion that is the counterion of a weak acid will have a pH greater than 7. In a sodium acetate solution, the sodium ions are pH neutral, whereas the acetate ions are basic and accept protons from the water molecules.
Some salts contain a cation and anion that are counterions of a weak acid and weak base, respectively. The solution will be acidic if the Ka of the cation is larger than the Kb of the anion; basic if the Kb of the anion is larger than the Ka of the cation; or neutral if the Ka and the Kb are equal.
In a solution of ammonium nitrite, the Ka for ammonium is higher than the Kb for nitrite ions; therefore, the solution will be acidic.
The exact pH of a salt solution containing an acidic or basic ion can be determined using the dissociation constant for the conjugate acid or base of that ion.
The pH of 0.35 M sodium cyanide can be determined by calculating the Kb of cyanide from the Ka of its conjugate acid – hydrocyanic acid – and setting up an ICE table to determine the equilibrium concentration.
As sodium cations do not react with water, they do not affect the pH and can be omitted. In contrast, cyanide ions accept protons from water molecules and generate hydroxide ions.
The Kb for cyanide ions can be determined using the equation: Ka × Kb = KW. As the Ka for hydrocyanic acid is 4.90 × 10−10, the Kb for cyanide ions is 2.04 × 10−5.
The Kb is equal to the concentration of hydrocyanic acid times the concentration of hydroxide ions divided by the concentration of cyanide ions.
An ICE table can be constructed to express the initial and equilibrium concentrations. Substituting the equilibrium concentrations into the expression, Kb equals x times x divided by 0.35, which can be verified later by the 5% rule.
Upon solving the equation, x equals 2.7 × 10−3 molar. As x is only 0.77% of 0.35 M, the approximation 0.35 minus x is equal to 0.35 is valid.
The pOH of the solution can be calculated by taking the negative log of 2.7 × 10−3 M, which equals 2.57.
The pH of the solution is determined using the equation pH plus pOH is equal to 14; therefore, the pH of this sodium cyanide solution is 11.43.
15.11:
Determining the pH of Salt Solutions
The pH of a salt solution is determined by its component anions and cations. Salts that contain pH-neutral anions and the hydronium ion-producing cations form a solution with a pH less than 7. For example, in ammonium nitrate (NH4NO3) solution, NO3− ions do not react with water whereas NH4+ ions produce the hydronium ions resulting in the acidic solution. In contrast, salts that contain pH-neutral cations and the hydroxide ion-producing anions form a solution with a pH greater than 7. For example, in sodium fluoride (NaF) solution, the Na+ is pH-neutral but the F– produces the hydroxide ions and forms the basic solution. The counterions of a strong acid or base are pH-neutral and salts formed by such counterions form a neutral solution with a pH equal to 7. For example, in KBr, The K+ cation is inert and will not affect pH. The bromide ion is the conjugate base of a strong acid, and so it is of negligible base strength (no appreciable base ionization). The solution is neutral.
Some salts contain both an acidic cation and a basic anion. The overall acidity or basicity of a solution is determined by the relative strength of the cation and anion, which can be compared using Ka and Kb. For example, in NH4F, the NH4+ ion is acidic and the F− ion is basic (conjugate base of the weak acid HF). Comparing the two ionization constants: Ka of NH4+ is 5.6 × 10−10 and the Kb of F− is 1.6 × 10−11, so the solution is acidic, since Ka > Kb.
Calculating the pH of an Acidic Salt Solution
Aniline is an amine that is used to manufacture dyes. It is isolated as anilinium chloride, [C6H5NH3+]Cl, a salt prepared by the reaction of the weak base aniline and hydrochloric acid. What is the pH of a 0.233 M solution of anilinium chloride?
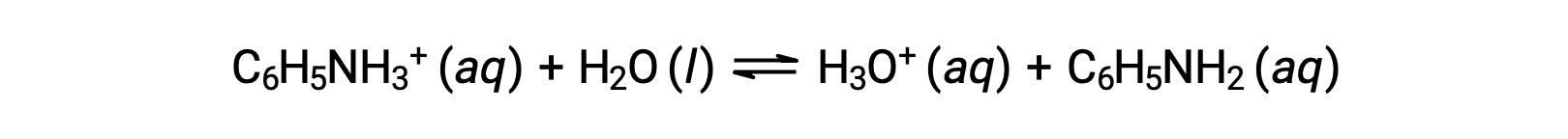
The Ka for anilinium ion is derived from the Kb for its conjugate base, aniline:
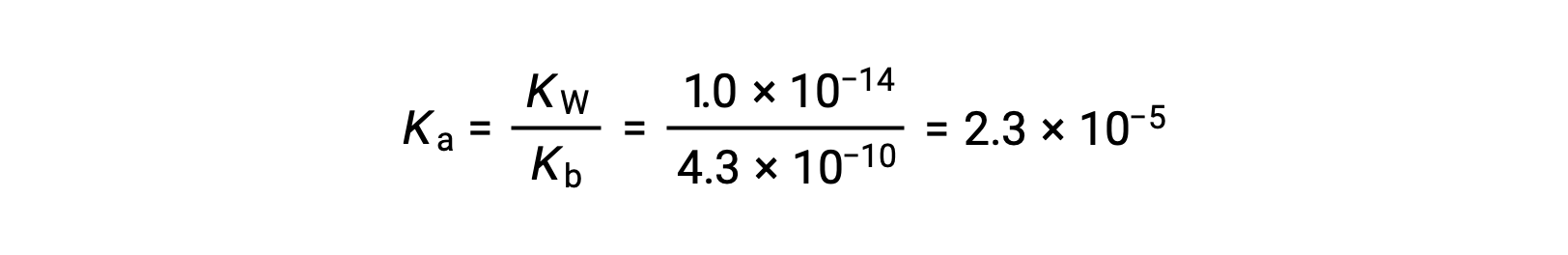
Using the provided information, an ICE table for this system is prepared:
| C6H5NH3+ (aq) | H3O+ (aq) | C6H5NH2 (aq) | |
| Initial Concentration (M) | 0.233 | ~0 | 0 |
| Change (M) | −x | +x | +x |
| Equilibrium Concentration (M) | 0.233 − x | x | x |
Substituting these equilibrium concentration terms into the Ka expression gives
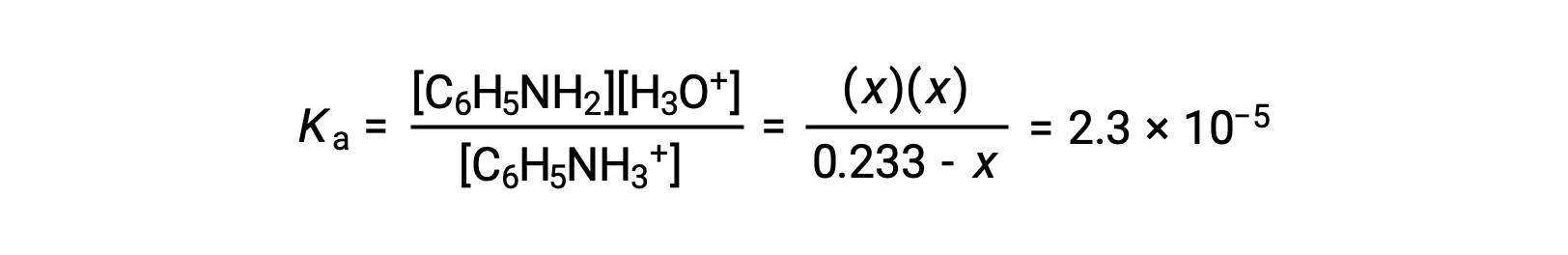
Assuming x << 0.233, the equation is simplified and solved for x:
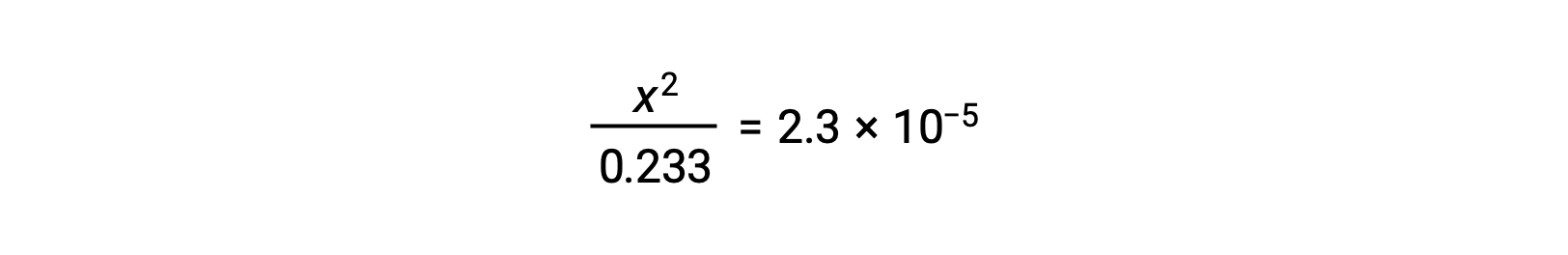

The ICE table defines x as the hydronium ion molarity, and so the pH is computed as
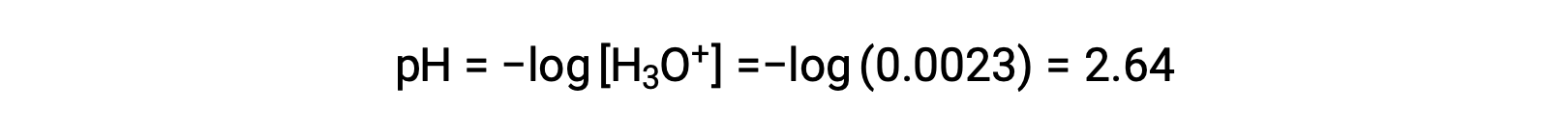
Hydrolysis of [Al(H2O)6]3+
Calculate the pH of a 0.10 M solution of aluminum chloride, which dissolves completely to give the hydrated aluminum ion [Al(H2O)6]3+ in solution.
The equation for the reaction and Ka are:
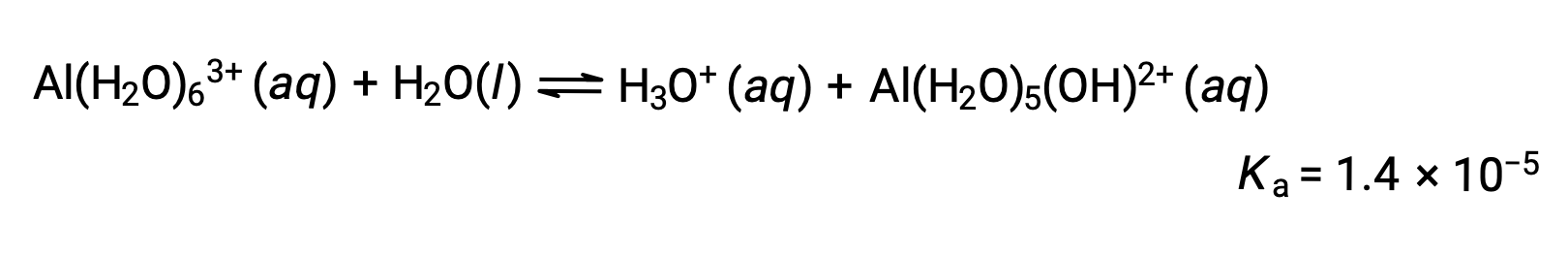
An ICE table with the provided information is
| Al(H2O)63+ (aq) | H3O+ (aq) | Al(H2O)5(OH)2+ (aq) | |
| Initial Concentration (M) | 0.10 | ~0 | 0 |
| Change (M) | −x | +x | +x |
| Equilibrium Concentration (M) | 0.10 − x | x | x |
Substituting the expressions for the equilibrium concentrations into the equation for the ionization constant yields:
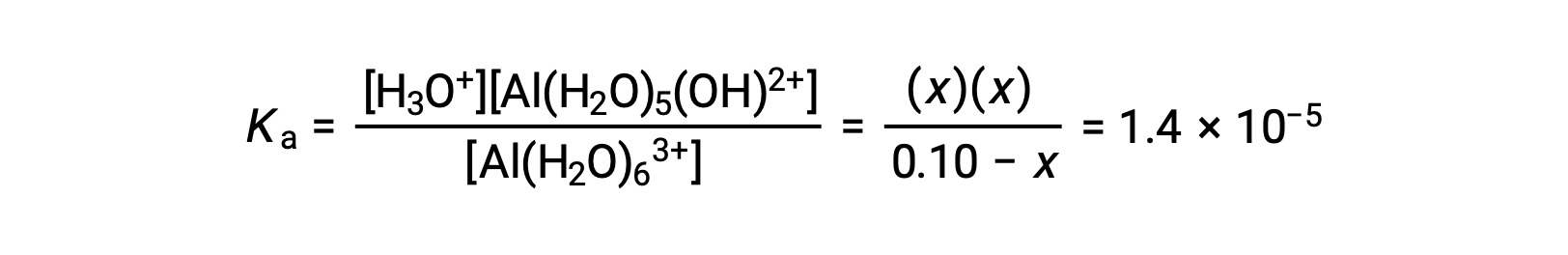
Assuming x << 0.10 and solving the simplified equation gives:
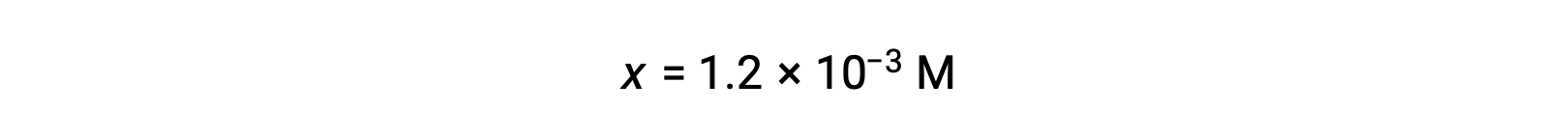
The ICE table defined x as equal to the hydronium ion concentration, and so the pH is calculated to be 2.92, and the solution is acidic.
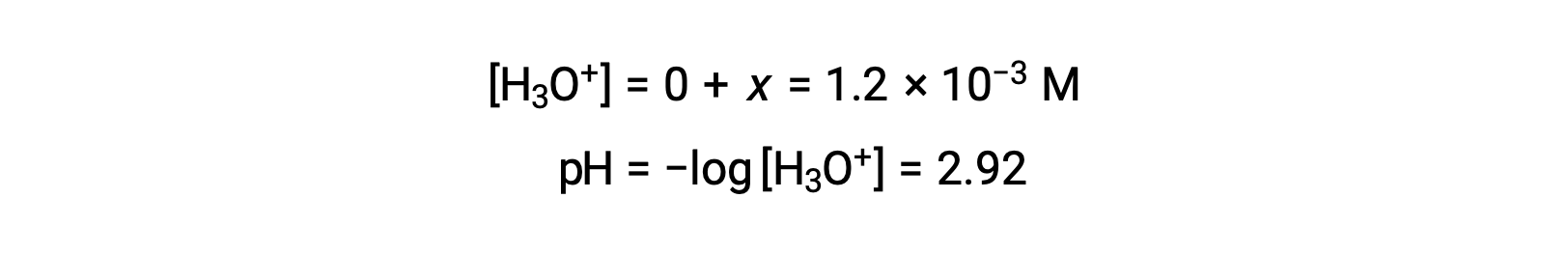
This text is adapted from Openstax, Chemistry 2e, Section 14.4: Hydrolysis of Salts.
