16.12:
Formation of Complex Ions
Metal ions are always hydrated in aqueous solutions. The water molecules act as Lewis bases, sharing their lone pair of electrons with the metal ions, which behave as Lewis acids.
When a Lewis base stronger than water is added, it displaces the water molecules and surrounds the central metal ion, forming a complex ion. The molecule or ion acting as the Lewis base is called a ligand.
In hexammine cobalt(III) chloride, hexammine cobalt is a complex ion, where the 6 ammonia molecules are the ligands that octahedrally surround the central cobalt ion.
Because transition metal ions have a high charge density and empty d orbitals to accommodate shared electrons, they are particularly prone to forming complex ions.
The equilibrium constant for the reaction between the metal ion and the ligand is called the formation constant, Kf. The larger the value of Kf, the more stable the complex ion.
Forming such stable complex ions often increases the solubility of sparingly soluble metal salts.
Consider silver sulfide, which exists in solution in an equilibrium of aqueous ions and undissolved solid.
If silver sulfide is added to sodium cyanide solution, the silver ions combine with cyanide to form the complex ion dicyanoargentate.
If 0.20 moles of silver sulfide is added to one liter of a 0.90 M sodium cyanide solution, the equilibrium concentration of silver ions, x, can be calculated from an ICE table.
The initial concentrations of silver, cyanide, and dicyanoargentate ions are 0.20 M, 0.90 M, and 0, respectively.
Because of the high Kf, and the much higher concentration of cyanide compared to silver ions, essentially all the silver ions are converted to dicyanoargentate ions.
One aqueous silver ion reacts with 2 cyanide ions to form dicyanoargentate. So, the change in molar concentration of cyanide ions will be 2 × 0.20, or 0.40 M.
Thus, at equilibrium the concentration of dicyanoargentate ions can be assumed to be the same as the initial concentration of silver, while cyanide ion concentration would be 0.90 − 0.40 M, or 0.50 M.
Substituting these values into the expression for Kf yields 0.2 molar divided by x times 0.5 squared. When the expression is solved for x, the resulting concentration is 8.0 × 10−22 M.
The very small equilibrium concentration of silver ions indicates that the formation of complex ions depletes free silver ions from the solution.
This drives the silver sulfide solubility equilibrium towards the ions, allowing more solid to dissolve.
16.12:
Formation of Complex Ions
A type of Lewis acid-base chemistry involves the formation of a complex ion (or a coordination complex) comprising a central atom, typically a transition metal cation, surrounded by ions or molecules called ligands. These ligands can be neutral molecules like H2O or NH3, or ions such as CN− or OH−. Often, the ligands act as Lewis bases, donating a pair of electrons to the central atom. These types of Lewis acid-base reactions are examples of a broad subdiscipline called coordination chemistry—the topic of another chapter in this text.
The equilibrium constant for the reaction of a metal ion with one or more ligands to form a coordination complex is called a formation constant (Kf) (sometimes called a stability constant). For example, the complex ion [Cu(CN)2]− is produced by the reaction
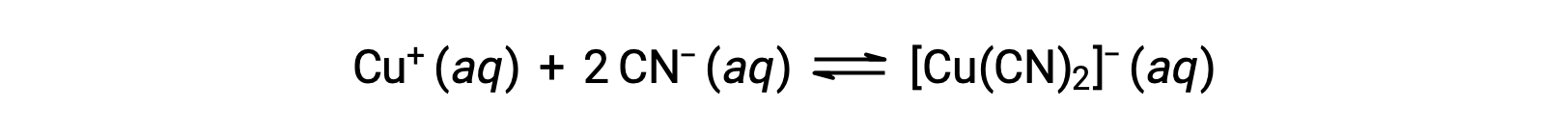
The formation constant for this reaction is
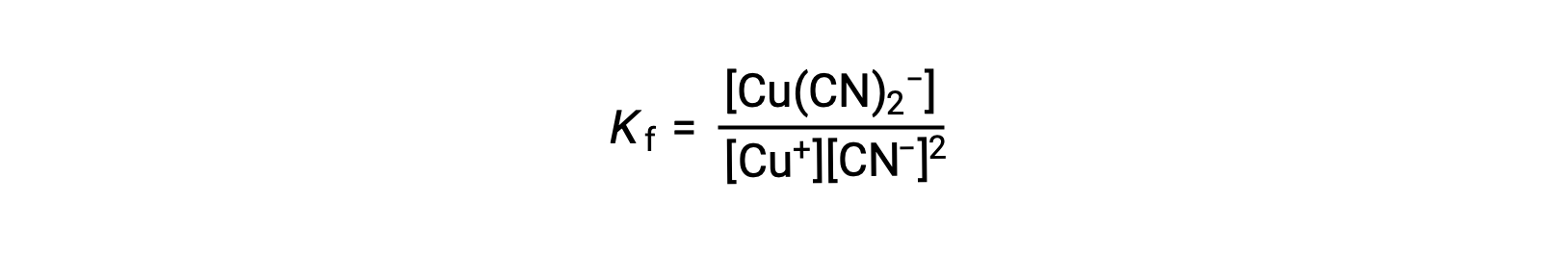
Alternatively, the reverse reaction (decomposition of the complex ion) can be considered, in which case the equilibrium constant is a dissociation constant (Kd). As per the relation between equilibrium constants for reciprocal reactions described, the dissociation constant is the mathematical inverse of the formation constant, Kd = Kf−1.
As an example of dissolution by complex ion formation, consider what happens when aqueous ammonia is added to a mixture of silver chloride and water. Silver chloride dissolves slightly in water, giving a small concentration of Ag+ ([Ag+] = 1.3 × 10−5 M):
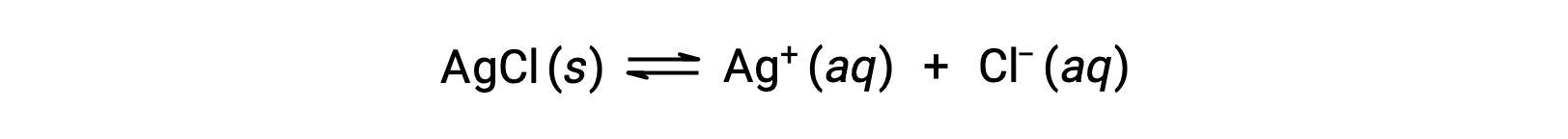
However, if NH3 is present in the water, the complex ion, [Ag(NH3)2]+, can form according to the equation:
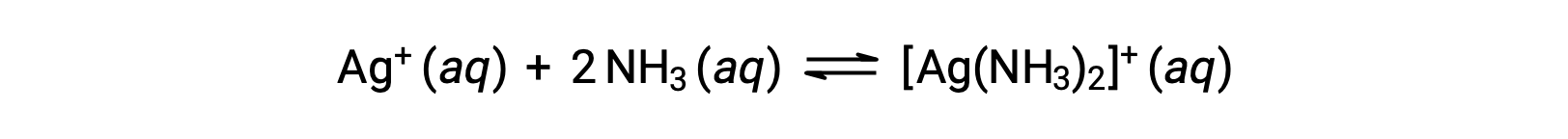
This text is adapted from Openstax, Chemistry 2e, Section 15.2: Lewis Acids and Bases.
Suggested Reading
- Xie, Feng, and David B. Dreisinger. "Leaching of silver sulfide with ferricyanide–cyanide solution." Hydrometallurgy 88, no. 1-4 (2007): 98-108.
- Glueck, A. R. "Desalination by an ion exchange-precipitation-complex process." Desalination 4, no. 1 (1968): 32-37.
- Shakhashiri, Bassam Z., Glen E. Dirreen, and Fred Juergens. "Solubility and complex ion equilibria of silver (I) species in aqueous solution." Journal of Chemical Education 57, no. 11 (1980): 813.
