16.13:
Precipitation of Ions
If the solutions of two ionic compounds are mixed, such as sodium iodide and lead(II) nitrate, the cations from one solution can combine with the anions from the other.
As one of the cross products—sodium nitrate— is water soluble, sodium and nitrate ions continue to remain in solution, while lead and iodide ions form a lead iodide precipitate.
The reaction quotient, Q, of the dissolution reaction is equal to the product of the concentrations of lead and iodide ions.
Unlike the solubility product Ksp, the reaction quotient involves ion concentrations at any stage, not just at equilibrium.
The values of Q and Ksp can be compared to predict if a precipitation reaction will occur.
Consider a dropwise addition of sodium iodide solution to the lead nitrate solution.
In the beginning, Q is less than Ksp, and both lead and iodide ions are in solution with the sodium and nitrate ions. This is an unsaturated solution.
As more sodium iodide is added, the concentration of iodide ions continues to increase. The reaction has reached equilibrium when Q = Ksp. At this stage, a small amount of solid lead iodide is in dynamic equilibrium with the ions, forming a saturated solution.
Further addition of sodium iodide makes Q > Ksp, and the reaction shifts towards the precipitate. This is a supersaturated solution, where precipitation continues until the ion concentrations are lowered to their equilibrium values.
For instance, suppose the mixing of sodium iodide and lead(II) nitrate solutions results in a solution that contains 1.6 × 10−4 M lead ions and 4.0 × 10−4 M iodide ions.
Here, Q = 2.6 × 10−11, while Ksp for lead iodide is 1.4 × 10−8. Because Q < Ksp, lead iodide will not precipitate.
Predicting precipitation reactions can be very useful during the separation of ionic compounds.
Consider a solution with two metal ions— lead(II) and copper(II). If hydrochloric acid is added to this solution, lead(II) chloride precipitates because it has a small Ksp, while copper remains in solution as copper(II) chloride is highly soluble in water.
This technique is called selective precipitation.
16.13:
Precipitation of Ions
Predicting Precipitation
The equation that describes the equilibrium between solid calcium carbonate and its solvated ions is:
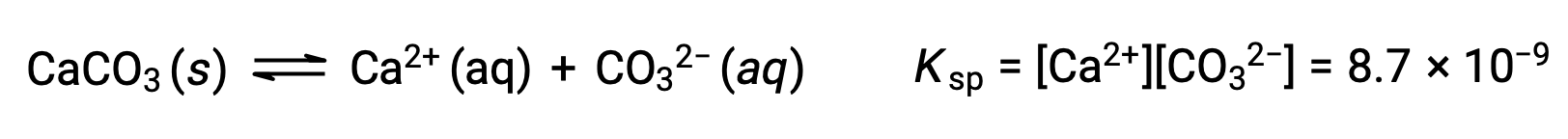
It is important to realize that this equilibrium is established in any aqueous solution containing Ca2+ and CO32– ions, not just in a solution formed by saturating water with calcium carbonate. Consider, for example, mixing aqueous solutions of the soluble compounds sodium carbonate and calcium nitrate. If the concentrations of calcium and carbonate ions in the mixture do not yield a reaction quotient, Q, that exceeds the solubility product, Ksp, then no precipitation will occur. If the ion concentrations yield a reaction quotient greater than the solubility product, then precipitation will occur, lowering those concentrations until equilibrium is established (Q = Ksp). The comparison of Q to Ksp to predict precipitation is an example of the general approach to predicting the direction of a reaction. For the specific case of solubility equilibria:
Q < Ksp: the reaction proceeds in the forward direction (solution is not saturated; no precipitation observed)
Q > Ksp: the reaction proceeds in the reverse direction (solution is supersaturated; precipitation will occur)
In solutions containing two or more ions that may form insoluble compounds with the same counter ion, an experimental strategy called selective precipitation may be used to remove individual ions from the solution. By increasing the counter ion concentration in a controlled manner, ions in solution may be precipitated individually, assuming their compound solubilities are adequately different. In solutions with equal concentrations of target ions, the ion forming the least soluble compound will precipitate first (at the lowest concentration of counter ion). The other ions subsequently precipitate as their compound’s solubilities are reached.
Precipitation of Silver Halides
A solution contains 0.00010 mol of KBr and 0.10 mol of KCl per liter. AgNO3 is gradually added to this solution. Which forms first, solid AgBr or solid AgCl?
The two equilibria involved are:
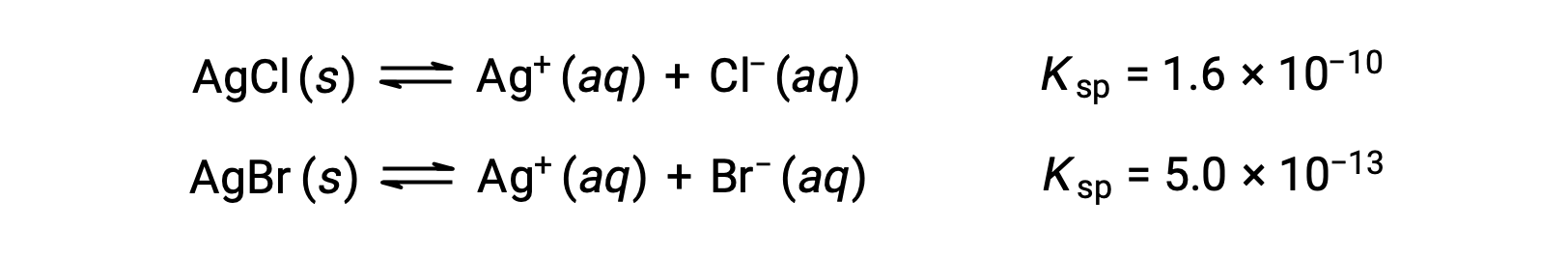
If the solution contained about equal concentrations of Cl– and Br–, then the silver salt with the smaller Ksp (AgBr) would precipitate first. The concentrations are not equal, however, so the [Ag+] at which AgCl begins to precipitate and the [Ag+] at which AgBr begins to precipitate must be calculated. The salt that forms at the lower [Ag+] precipitates first.
AgBr precipitates when Q = Ksp for AgBr
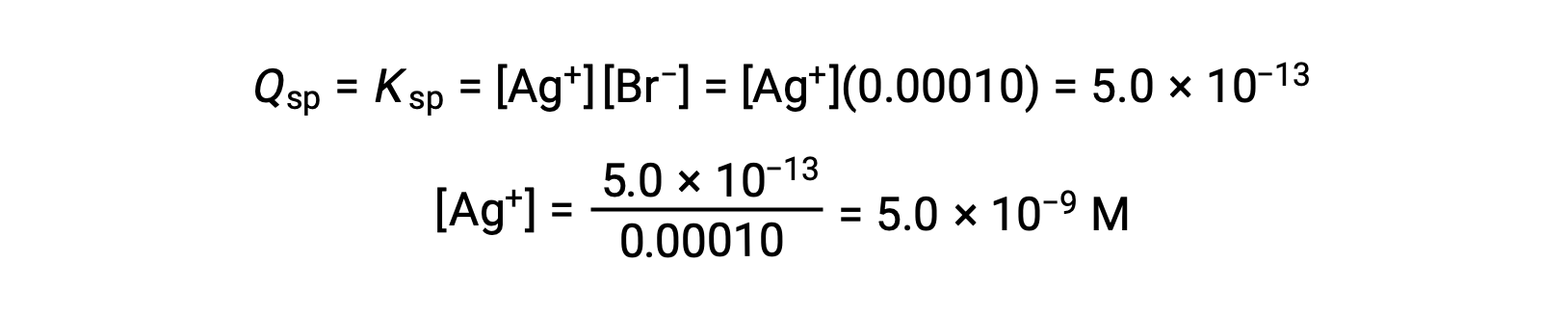
AgBr begins to precipitate when [Ag+] is 5.0 × 10−9 M.
For AgCl:
AgCl precipitates when Q equals Ksp for AgCl (1.6 × 10-10). When [Cl–] = 0.10 M:
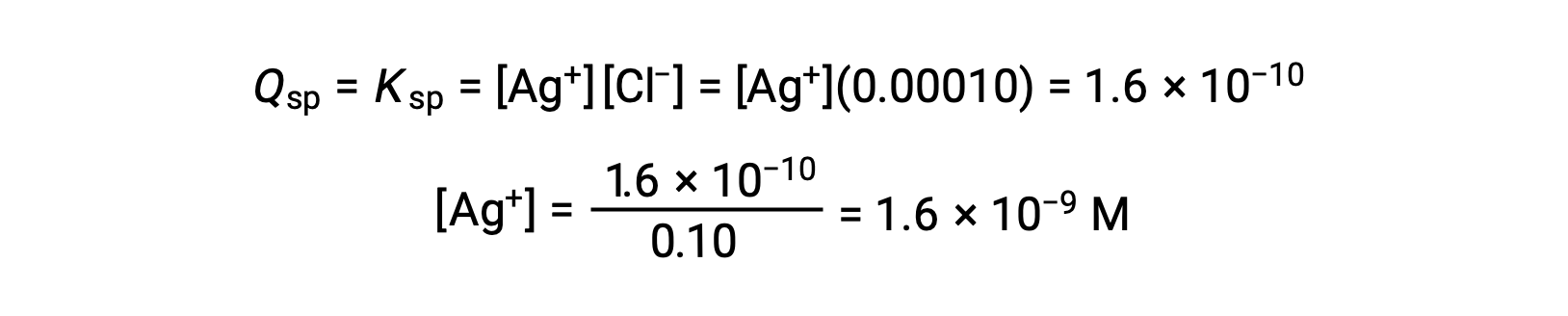
AgCl begins to precipitate when [Ag+] is 1.6 × 10-9 M.
AgCl begins to precipitate at a lower [Ag+] than AgBr, so AgCl begins to precipitate first. Note the chloride ion concentration of the initial mixture was significantly greater than the bromide ion concentration, and so silver chloride precipitated first despite having a Ksp greater than that of silver bromide.
This text is adapted from Openstax, Chemistry 2e, Chapter 15.1: Precipitation and Dissolution.
Suggested Reading
- Firsching, Ferdinand Henry. "Selective precipitation of silver halides from homogeneous solution. Separation of iodide, bromide, and chloride using volatilization of ammonia." Analytical Chemistry 32, no. 13 (1960): 1876-1878.
- Reynolds, John P. "Ksp experiment: The solubility product for barium hydroxide." Journal of Chemical Education 52, no. 8 (1975): 521.
- Hou, Miaolin, and George L. Baughman. "Predicting the precipitation of acid and direct dyes in natural waters." Dyes and pigments 18, no. 1 (1992): 35-46.
