16.12:
錯体イオンの形成
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Core
Chemistry
Formation of Complex Ions
Metal ions are always hydrated in aqueous solutions. The water molecules act as Lewis bases, sharing their lone pair of electrons with the metal ions, which behave as Lewis acids. When a Lewis base stronger than water is added, it displaces the water molecules and surrounds the central metal ion, forming a complex ion. The molecule or ion acting as the Lewis base is called a ligand. In hexammine cobalt(III) chloride, hexammine cobalt is a complex ion, where the 6 ammonia molecules are the ligands that octahedrally surround the central cobalt ion. Because transition metal ions have a high charge density and empty d orbitals to accommodate shared electrons, they are particularly prone to forming complex ions. The equilibrium constant for the reaction between the metal ion and the ligand is called the formation constant, Kf. The larger the value of Kf, the more stable the complex ion. Forming such stable complex ions often increases the solubility of sparingly soluble metal salts. Consider silver sulfide, which exists in solution in an equilibrium of aqueous ions and undissolved solid. If silver sulfide is added to sodium cyanide solution, the silver ions combine with cyanide to form the complex ion dicyanoargentate. If 0.20 moles of silver sulfide is added to one liter of a 0.90 M sodium cyanide solution, the equilibrium concentration of silver ions, x, can be calculated from an ICE table. The initial concentrations of silver, cyanide, and dicyanoargentate ions are 0.20 M, 0.90 M, and 0, respectively. Because of the high Kf, and the much higher concentration of cyanide compared to silver ions, essentially all the silver ions are converted to dicyanoargentate ions. One aqueous silver ion reacts with 2 cyanide ions to form dicyanoargentate. So, the change in molar concentration of cyanide ions will be 2 × 0.20, or 0.40 M. Thus, at equilibrium the concentration of dicyanoargentate ions can be assumed to be the same as the initial concentration of silver, while cyanide ion concentration would be 0.90 − 0.40 M, or 0.50 M. Substituting these values into the expression for Kf yields 0.2 molar divided by x times 0.5 squared. When the expression is solved for x, the resulting concentration is 8.0 × 10−22 M. The very small equilibrium concentration of silver ions indicates that the formation of complex ions depletes free silver ions from the solution. This drives the silver sulfide solubility equilibrium towards the ions, allowing more solid to dissolve.
16.12:
錯体イオンの形成
ルイス酸塩基化学の一種である錯体イオン(または配位錯体)は、中心原子(典型的には遷移金属陽イオン)が配位子と呼ばれるイオンや分子に囲まれて構成されたものです。これらの配位子は、H2OやNH3のような中性分子であったり、CN−やOH−のようなイオンの場合もあります。多くの場合、配位子はルイス塩基として働き、中心原子に一対の電子を供与します。このようなルイス酸塩基反応は、配位化学と呼ばれる幅広い分野の一例であり、この記事の別の章のテーマでも紹介しています。
金属イオンが1つ以上の配位子と反応して配位錯体を形成する際の平衡定数を生成定数(Kf)と呼ぶ(安定定数と呼ばれることもある)。例えば、錯体イオン[Cu(CN)2]−は次のような反応で生成します。
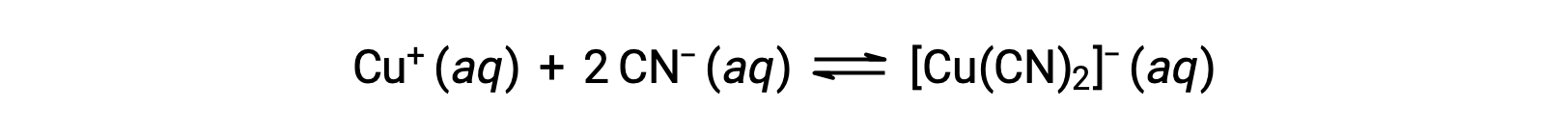
この反応の生成定数は、
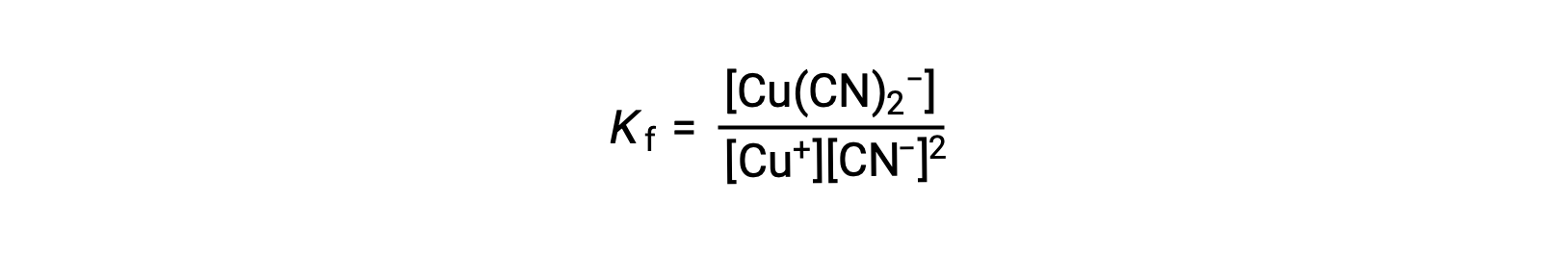
また、逆の反応(錯イオンの分解)も考えられ、その場合の平衡定数は解離定数(Kd)と呼ばれます。前述の相互反応における平衡定数の関係のように、解離定数は生成定数の逆数です。(Kd=Kf−1)
錯イオン形成による溶解の例として、塩化銀と水の混合物にアンモニア水を加えた場合を考えましょう。塩化銀は水にわずかに溶解し、わずかな濃度のAg+([Ag+]=1.3 × 10−5 M)が得られます。
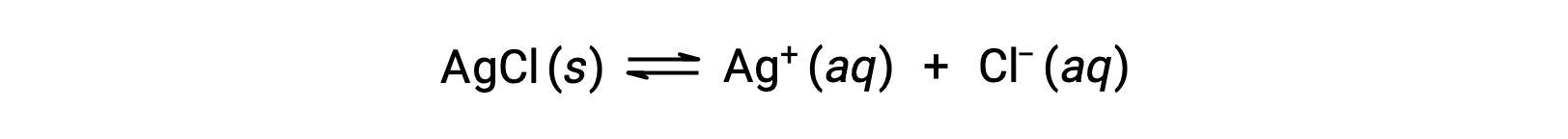
しかし、水中にNH3が存在すると、次式に従って錯イオンである[Ag(NH3)2]+が形成されます。
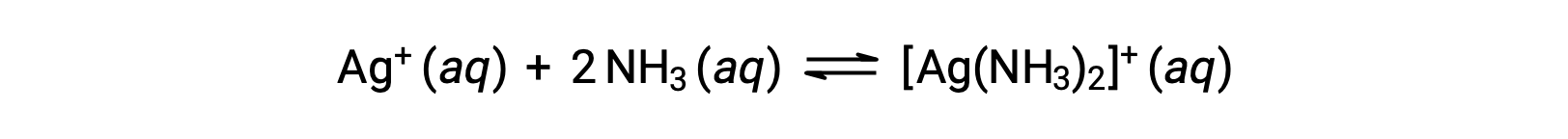
上記の文章は以下から引用しました。Openstax, Chemistry 2e, Section 15.2: Lewis Acids and Bases.
Suggested Reading
- Xie, Feng, and David B. Dreisinger. "Leaching of silver sulfide with ferricyanide–cyanide solution." Hydrometallurgy 88, no. 1-4 (2007): 98-108.
- Glueck, A. R. "Desalination by an ion exchange-precipitation-complex process." Desalination 4, no. 1 (1968): 32-37.
- Shakhashiri, Bassam Z., Glen E. Dirreen, and Fred Juergens. "Solubility and complex ion equilibria of silver (I) species in aqueous solution." Journal of Chemical Education 57, no. 11 (1980): 813.
