16.15:
Acid-Base Titration Curves
Acid-base titration can be performed to determine an unknown concentration of an acid using a known concentration of a base or vice versa.
When titrating an acid with a base, the titrant is gradually added to the acid, raising the pH. The pH change is plotted against the volume of the base to create a titration or pH curve.
The titration curves are S-shaped, though differences exist depending on whether the acid is strong or weak.
The pH at the start of the titration will be acidic but higher for a weak acid, assuming equal initial concentrations of acid.
The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution.
In the case of a strong acid, the equivalence point is reached when a strong base neutralizes all the hydronium ions produced by the strong acid; therefore, it always occurs at pH 7.
In contrast, a weak acid’s equivalence point occurs at a pH greater than 7 and is reached when all the weak acid is converted into its conjugate base.
Near the equivalence point, there is a sudden jump in the pH of the solution. However, in a weak acid titration, this rapid pH shift is not as steep as in a strong acid titration.
The pH after the equivalence point on a titration curve for both weak and strong acids gradually increases due to excess strong base.
Other features of a weak acid titration curve, but not a strong one, are the buffer region and the half-equivalence point.
When a strong base is added, a weak acid produces its conjugate base creating a buffer. The half-equivalence point lies within this buffer region.
This point is when half of the volume of base needed to reach the equivalence point has been added. Here, the concentration of weak acid equals its conjugate base, and the pH of the solution equals the pKa.
The titration curves of a strong or a weak base with a strong acid as the titrant is an inverted S-shaped, where the pH starts high and decreases with the addition of the strong acid.
16.15:
Acid-Base Titration Curves
A titration curve is a plot of some solution property versus the amount of added titrant. For acid-base titrations, solution pH is a useful property to monitor because it varies predictably with the solution composition and, therefore, may be used to monitor the titration’s progress and detect its endpoint. Acid-base titration can be performed with a strong acid and a strong base, a strong acid and a weak base, or a strong base and a weak acid.
For a titration carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M of a strong base NaOH, its titration curve can be seen in red in Figure 1a. For a titration carried out for 25.00 mL of 0.100 M CH3COOH (weak acid) with 0.100 M NaOH, its titration curve can be seen in yellow in Figure 1b.
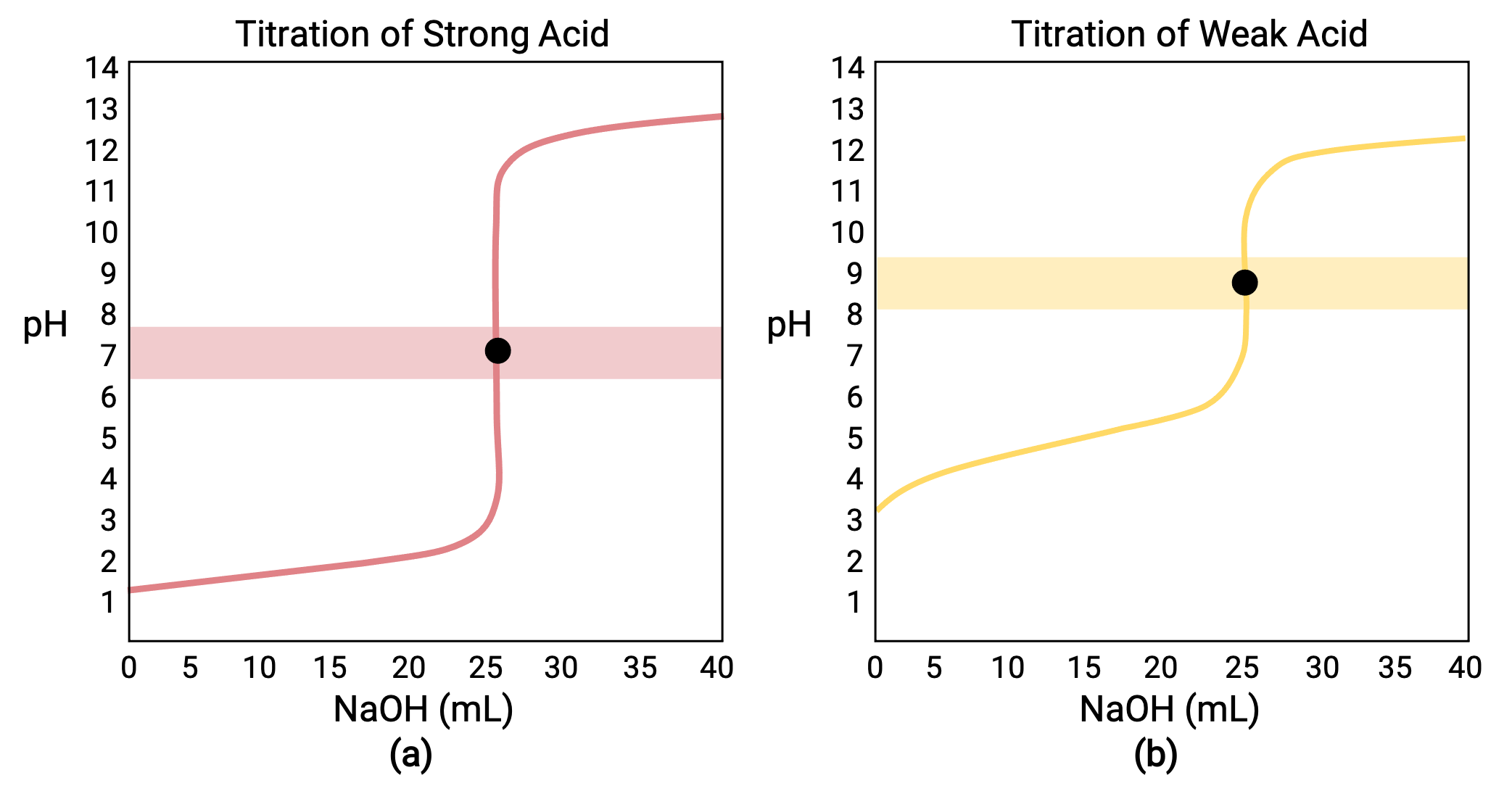
Figure 1 (a) The titration curve for the titration of 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M NaOH (strong base) has an equivalence point of 7.00 pH. (b)The titration curve for the titration of 25.00 mL of 0.100 M acetic acid (weak acid) with 0.100 M NaOH (strong base) has an equivalence point of 8.72 pH.
The titration of a strong or weak base with a strong acid has a similar S-shaped curve; however, the curve is inverted as the pH will start in the basic region and decrease with the addition of the strong acid. The titration of weak acid or base can also be used to determine the Ka or Kb, respectively.
The four stages of a titration:
- Initial state (added titrant volume = 0 mL): pH is determined by the acid being titrated; because the two acid samples are equally concentrated, the weak acid will exhibit a greater initial pH
- Pre-equivalence point (0 mL < V < 25 mL): solution pH increases gradually and the acid is consumed by reaction with added titrant; composition includes unreacted acid and the reaction product, its conjugate base
- Equivalence point (V = 25 mL): a drastic rise in pH is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak acid sample), with pH determined by ionization of the conjugate base of the acid
- Post-equivalence point (V > 25 mL): pH is determined by the amount of excess strong base titrant added; since both samples are titrated with the same titrant, both titration curves appear similar at this stage.
This text is adapted from Openstax, Chemistry 2e, Section 14.7: Acid-base Titrations.
Suggested Reading
- Ramette, Richard W. "TITRATE: A Learning Tool for Acid-Base Titrations." (1994): 238. https://pubs.acs.org/doi/pdf/10.1021/ed071p238.2
