17.7:
自由エネルギーに対する温度の影響
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Core
Chemistry
Effects of Temperature on Free Energy
For a reaction to be spontaneous at constant temperature and pressure, the change in Gibbs free energy, ΔG, must be less than zero. The sign of ΔG depends on the signs and the relative values of enthalpy, entropy, and temperature. Enthalpy favors spontaneity when the reaction releases heat to the surroundings, while entropy favors spontaneity when there is more disorder in the system. If ΔH is negative and ΔS is positive, as in the reaction between sodium hydroxide and hydrochloric acid, ΔG is negative at all temperatures. Thus, exothermic reactions—where the entropy of the system increases—are always spontaneous. If both ΔH and ΔS are negative, ΔG depends on the temperature. Consider the freezing of water into ice, an exothermic reaction where the entropy of the system decreases. At temperatures below the freezing point of water, the water will freeze spontaneously, releasing heat and becoming more ordered. Thus, reactions with negative enthalpy and entropy changes are spontaneous only at low temperatures. ΔG is also dependent on temperature if both ΔH and ΔS are positive. A common example is a chemical cold pack, where solid ammonium nitrate dissolves in water, that absorbs heat from the surroundings. This endothermic reaction proceeds spontaneously at room temperature due to the increase in disorder of the system. Thus, reactions with positive enthalpy and entropy changes are spontaneous only at higher temperatures. If the temperature were lowered such that the TΔS becomes smaller than ΔH, ΔG would be positive, and the reaction would become nonspontaneous. When ΔH is positive and ΔS is negative, ΔG is always positive, and the reaction is nonspontaneous at all temperatures.
17.7:
自由エネルギーに対する温度の影響
あるプロセスの自発性は、系の温度に依存します。例えば、相転移は、物質の温度に応じて、どちらかの方向に自然に進行します。同様に、化学反応も温度に依存した自発性を示すものがあります。この概念を説明するために、自由エネルギーの変化とプロセスのエンタルピーおよびエントロピー変化との関係式を考えます。
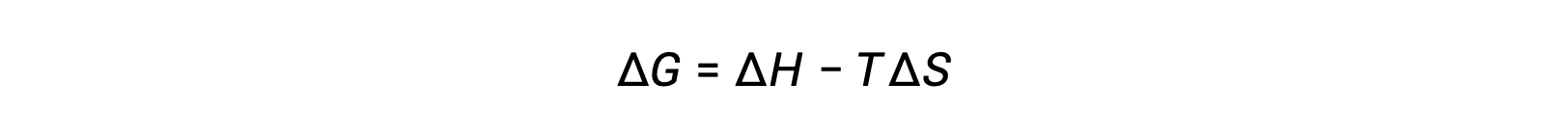
自由エネルギー変化の符号に反映されるプロセスの自発性は、エンタルピーおよびエントロピー変化の符号と、絶対温度によって決定されます。Tは絶対温度(K)なので、正の値しかとりません。したがって、エンタルピー変化とエントロピー変化の符号については、4つの可能性があります。
- ΔHとΔSの両方が正の場合 この条件は、系のエントロピーの増加を伴う吸熱過程を表しています。この場合、TΔS項の大きさが ΔHよりも大きければ ΔGは負の値となります。TΔS項の大きさが ΔHよりも小さい場合は、自由エネルギーの変化は正になります。このような過程は高温では自発的であり、低温では非自発的です。
- ΔHと ΔSの両方が負の場合 この条件は、系のエントロピーの減少を伴う発熱過程を表しています。この場合、TΔS項の大きさが ΔHよりも小さければ ΔGは負になります。TΔS項’の大きさがΔHよりも大きければ、自由エネルギーの変化は正になります。このような過程は、低温では自発的であり、高温では非自発的です。
- ΔH が正で、 ΔSが負の場合 この条件は、系のエントロピーの減少を伴う吸熱過程を表しています。この場合、ΔGは温度に関係なく正となります。このような過程は、すべての温度で自発的に進行しません。
- ΔHが負で、ΔSが正の場合 この条件は、系のエントロピーの増加を伴う発熱過程を表しています。この場合、 ΔG は温度に関係なく負の値をとります。このような過程は、すべての温度で自発的に起こります。
上記の文章は以下から引用しました。Openstax, Chemistry 2e, Section 16.4: Free Energy.
