9.4:
Nuclear Binding Energy
Nuclear stability is best quantified in terms of nuclear binding energy.
Consider the helium-4 atom, which has two each of protons, neutrons, and electrons. The sum of the known masses of these particles is greater than the measured mass of neutral helium-4 by 0.0305 atomic mass units.
The difference between the calculated and experimentally measured atomic masses is called the mass defect. The large amount of energy released during the formation of helium-4 is the reason for this difference.
Einstein’s mass–energy equivalence helps to estimate the energy change associated with the loss in mass. Converting the mass to kilograms and solving the equation results in the base SI units for joules. It is evident that an enormous amount of energy accompanies the tiny change in mass.
The energy released when the nucleons bind together is the same as the energy required to break that nucleus into its constituent protons and neutrons and is called nuclear binding energy. For helium, this is 2.74 terajoules per mole.
Dividing by Avogadro's number gives 4.55 picojoules for the nuclear binding energy per helium nucleus. This is often expressed in electronvolts as well. For helium-4, this turns out to be 28.4 megaelectronvolts per nucleus. When divided by the number of nucleons, 4, this yields the nuclear binding energy per nucleon.
The plot of nuclear binding energy per nucleon versus mass number depicts the comparative stabilities of the nuclides. The elements with mass numbers ranging from 40 to 100 have the highest per-nucleon binding energy, and iron-56 has the lowest mass per nucleon.
To attain stability, heavy nuclei tend to fragment to midsize nuclei through an exothermic process called fission, whereas lighter nuclei combine through the fusion process
9.4:
Nuclear Binding Energy
The difference between the calculated and experimentally measured masses is known as the mass defect of the atom. In the case of helium-4, the mass defect indicates a “loss” in mass of 4.0331 amu – 4.0026 amu = 0.0305 amu. The loss in mass accompanying the formation of an atom from protons, neutrons, and electrons is due to the conversion of that mass into energy that is evolved as the atom forms. The nuclear binding energy is the energy produced when the atoms’ nucleons are bound together; this is also the energy needed to break a nucleus into its constituent protons and neutrons. The energy changes associated with nuclear reactions are vastly greater than are those for chemical reactions.
The conversion between mass and energy is most identifiably represented by the mass–energy equivalence equation as stated by Albert Einstein: E = mc2, where E is energy, m is mass of the matter being converted, and c is the speed of light in a vacuum. Using this mass–energy equivalence equation, the nuclear binding energy of a nucleus may be calculated from its mass defect. A variety of units are commonly used for nuclear binding energies, including electronvolts (eV), with 1 eV equaling the amount of energy necessary to move the charge of an electron across an electric potential difference of 1 volt: 1.602 × 10–19 J.
To calculate the binding energy from the mass defect, first, express the mass defect in g/mol. This is easily done considering the numerical equivalence of atomic mass (amu) and molar mass (g/mol) that results from the definitions of the amu and mole units. The mass defect for He-4 is therefore 0.0305 g/mol. To accommodate the units of the other terms in the mass–energy equation, the mass must be expressed in kilograms, since 1 J = 1 kg m2/s2. Converting grams into kilograms yields a mass defect of 3.05 × 10–5 kg/mol. Substituting this quantity into the mass–energy equivalence equation yields:
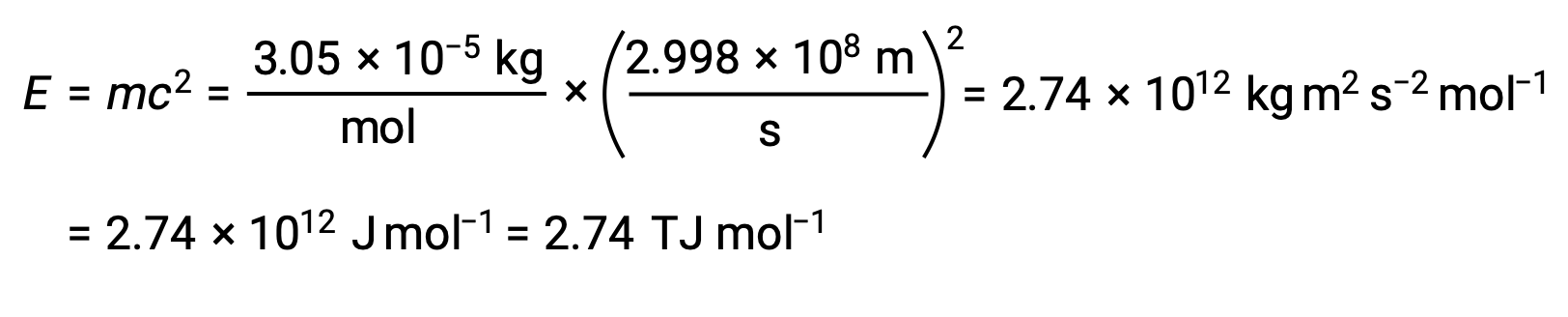
The binding energy for a single nucleus is computed from the molar binding energy using Avogadro’s number:
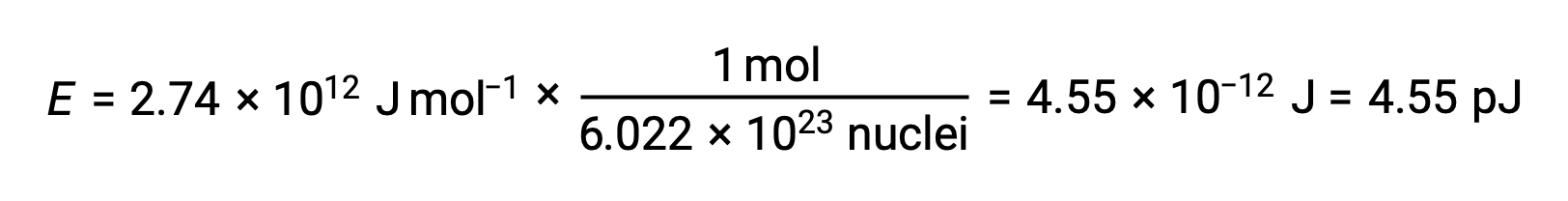
Recall that 1 eV = 1.602 × 10–19 J. Using the binding energy computed:
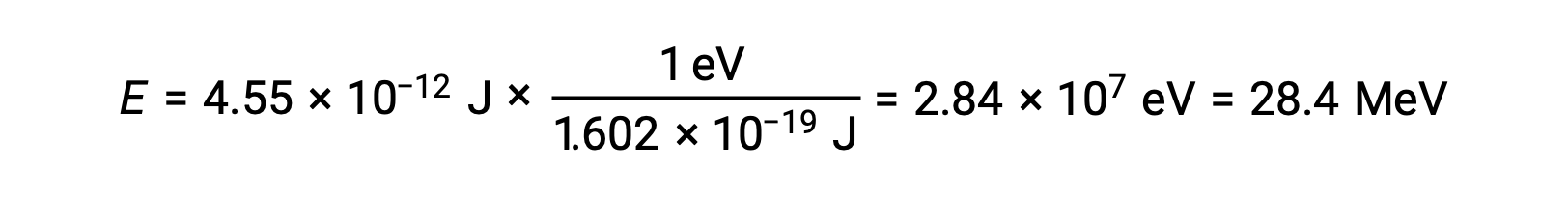
The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by the number of nucleons in the nucleus. For instance, the binding energy for a helium-4 nucleus is 28.4 MeV. The binding energy per nucleon for a helium-4 nucleus is therefore:
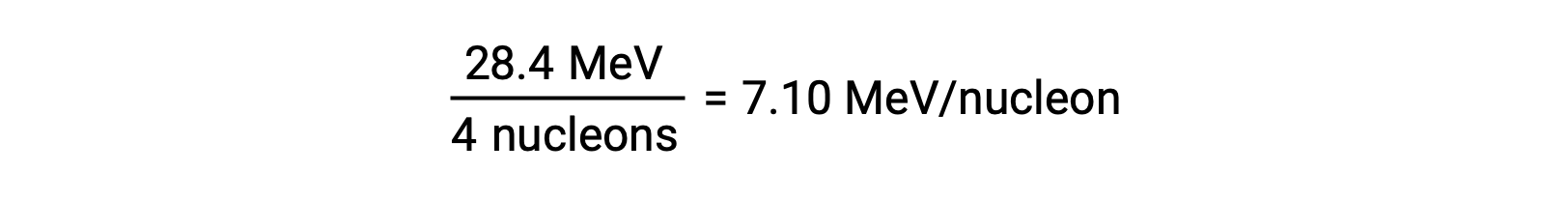
The binding energy per nucleon is largest for nuclides with a mass number of approximately 56.
This text is adapted from Openstax, Chemistry 2e, Section 21.1: Nuclear Structure and Stability.
