Ultrasound Assessment of Endothelial-Dependent Flow-Mediated Vasodilation of the Brachial Artery in Clinical Research
Summary
Endothelial dysfunction is associated with numerous disease states and is predictive of adverse cardiovascular events in humans. Flow-mediated vasodilation (FMD) is a non-invasive ultrasound method of evaluating endothelial function. Methodological choices and operator experience may affect results. A systematic approach to FMD in human studies is discussed here.
Abstract
The vascular endothelium is a monolayer of cells that cover the interior of blood vessels and provide both structural and functional roles. The endothelium acts as a barrier, preventing leukocyte adhesion and aggregation, as well as controlling permeability to plasma components. Functionally, the endothelium affects vessel tone.
Endothelial dysfunction is an imbalance between the chemical species which regulate vessel tone, thombroresistance, cellular proliferation and mitosis. It is the first step in atherosclerosis and is associated with coronary artery disease, peripheral artery disease, heart failure, hypertension, and hyperlipidemia.
The first demonstration of endothelial dysfunction involved direct infusion of acetylcholine and quantitative coronary angiography. Acetylcholine binds to muscarinic receptors on the endothelial cell surface, leading to an increase of intracellular calcium and increased nitric oxide (NO) production. In subjects with an intact endothelium, vasodilation was observed while subjects with endothelial damage experienced paradoxical vasoconstriction.
There exists a non-invasive, in vivo method for measuring endothelial function in peripheral arteries using high-resolution B-mode ultrasound. The endothelial function of peripheral arteries is closely related to coronary artery function. This technique measures the percent diameter change in the brachial artery during a period of reactive hyperemia following limb ischemia.
This technique, known as endothelium-dependent, flow-mediated vasodilation (FMD) has value in clinical research settings. However, a number of physiological and technical issues can affect the accuracy of the results and appropriate guidelines for the technique have been published. Despite the guidelines, FMD remains heavily operator dependent and presents a steep learning curve. This article presents a standardized method for measuring FMD in the brachial artery on the upper arm and offers suggestions to reduce intra-operator variability.
Introduction
The human vascular endothelium provides structural and functional roles within the body. In histological sections, the endothelium appears small, comprising a thin layer of cells 1-2 microns thick sitting atop a layer of smooth muscle cells (the media) and a thick layer of connective tissue (the adventitia). Taken as a whole, the endothelium provides a wide area for the exchange of information between the blood and vascular smooth muscle tissue. By one estimate, a cross sectional area of 700 m2 and a mass of 1,000-1,500 grams in a 70 kg man, is comparable in mass to the liver1. A healthy endothelium allows for mechanical to chemical signal transduction to maintain homeostasis of the blood vessel. Endothelial dysfunction is an imbalance of these mediators and the first step in vascular disease, present prior to histological evidence of atherosclerosis. A non-invasive, in vivo method for quantifying the vasodilatory function of human artery exists. This method, endothelium-dependent, flow-mediated vasodilation (FMD) is widely used in clinical trials.
The endothelium acts as a structural component of the vasculature and manufactures components of the extracellular matrix such as glycosaminoglycans and fibronectin2. Long term changes in blood flow and acute injury to the artery may lead to structural changes. Functionally, the vascular endothelial cells participate in regulation of vessel tone, inflammatory processes, antithrombosis, and anticoagulation. Endothelial cells affect vasoconstriction through endothelin while vasodilation is mediated by nitric oxide (NO), prostacyclin, and endothelial derived hyperpolarizing factor (EDHF)3-6.
Endothelial dysfunction is an impairment of any of these mediators and the first step in atherosclerosis. Not surprisingly, as a mechanism of disease, it is associated with a number of clinically important conditions such as coronary artery disease, hypertension and diabetes mellitus7-11. Importantly, endothelial dysfunction can be observed in individuals without diagnosed cardiovascular disease and is predictive of future cardiovascular events7,12,13. One measure of endothelial dysfunction, in combination with the Framingham score, can provide additional prognostic information above either measure alone14.
Measures of endothelial dysfunction may involve the direct infusion of a pharmacological agent. Intercoronary infusion of acetylcholine, for example, combined with quantitative angiography demonstrates vasodilation in subjects with an intact endothelium. However, individuals with endothelial damage experience paradoxical vasoconstriction.15 In peripheral arteries, infusion of a pharmacological agent with measurement of flow by gauge-strain plethysmography is possible16.
Agents that directly affect the endothelium and elicit a chemical signal are termed endothelium-dependent vasodilators. Acetylcholine, for example, acts on muscarinic receptors on endothelial cells, leading to increased intra-cellular calcium concentration, activation of nitric oxide synthase and vasodilation. Agents that affect vasodilation without involvement of the endothelium are called endothelium-independent agents. Nitroglycerin, for example, activates soluble guanyl cyclase and cyclic guanosine-3’,-5’-monophasphate (cGMP) which mediates vasodilation in the vessel wall through protein kinases regulating intracellular calcium concentrations17.
There is a non-invasive, in vivo method for quantifying endothelial dysfunction introduced by Celermajer and associates called “flow-mediated, endothelium-dependent vasodilation” (FMD)18. Briefly, changes to arterial blood flow open shear stress sensitive ion channels in the endothelium. The signal is tranduced via a second-messenger cascade and activates endothelial nitric oxide synthase (eNOS), generating NO. This species diffuses across the cell membrane to neighboring smooth muscle cells (SMC). Within the SMC, the signal is transduced, lowering intracellular calcium concentration and affecting vasorelaxation19. The diameter of the artery lumen increases, leading to an increase in blood flow consistent with the Hagen-Poiseullie equation. The effect of FMD may be abolished with administration of an NO synthase inhibitor such as mono-methylarginine (L-NMMA)20.
Celermajer et al.’s innovative work has allowed the use of high resolution B-mode ultrasound to assess the change in artery diameter during the reactive hyperemia that follows ischemia. In this technique, a human subject rests supine and the diameter of the brachial artery is measured in a longitudinal plane. A blood-pressure cuff is used to produce ischemia in the limb. Following release of the blood pressure cuff the diameter of the artery is measured again. The rapid change in shear stress is the stimulus for NO mediated vasodilation. A simple equation describes the change in the diameter relative to the baseline diameter (Equation 1). A full discussion of the parameters of this equation, hyperemia and baseline diameter, can be found in the Protocol and Results sections.
<!–Equation 1: Percent FMD
%FMD = 
In multiple studies, percent FMD has been found to predict cardiovascular events in patient with established cardiovascular disease21-24. A correlation between brachial artery percent FMD and coronary artery FMD was established by Anderson et al., demonstrating a link between peripheral measurements and the more clinically-relevant ischemic changes to the heart25. FMD does not demonstrate the maximum vasodilation of the vessel. To evaluate this, FMD can be followed by endothelium-dependent, nitroglycerin-mediated vasodilation of the same vessel.
There are technical issues affecting the measurement of percent FMD. Since the introduction of the technique, several studies showed a high degree of within-subject and inter-operator variability26. It has been shown that physiological factors such as cigarette smoking, antihypertensive medications, time of day, and fasting state affect percent FMD. Likewise, technical choices such as the position of the cuff relative to the site of measurement and duration of occlusion have been shown to affect the measurement27,28. Guidelines have been published that describe the current consensus and allow for standardization of technique between laboratories19,29.
Despite the evolving consensus on technique, flow-mediated vasodilation remains heavily operator dependent with a long learning curve. Corretti, for example, recommends the sonographer complete 100 scans under the supervision of an experienced investigator before operating independently. To maintain a level of adequate expertise, it is recommended the technician complete 100 scans annually. For investigators with a small sample population and limited resources, the learning curve presents a barrier to entry. This article will demonstrate a method for flow-mediated vasodilation of the brachial artery in the upper arm and offer technical suggestions to reduce intra-operator variability.
Protocol
The following procedure, developed as part of an investigator-initiated study, was reviewed and approved by the University of California, San Francisco (UCSF) Committee on Human Research (CHR) and all participants gave informed consent.
1. Equipment
- Use an EKG gated image capture system to record and analyze the FMD. Connect a Philips HD11 ultrasound to a desktop PC.
- Connect a video signal from the ultrasound with a special frame-grabber card on the PC.
- Relay an audio signal from the ultrasound to an EKG gating module which amplifies the signal. Carry the amplified signal to the PC, to allow the image capture software to identify and record images at a consistent point in the cardiac cycle. Generate the signal from the sharp deflection of the R-wave in the EKG.
- Use a 5-12 MHz linear array transducer to optimize resolution at the depth of the brachial artery.
2. Subject Preparation
- Ensure that participants fast and avoid exercise for 8 hr before the exam as well as avoid caffeine or nicotine for at least four fours. Ensure that participants avoid medications affecting vascular tone or cardiac output for four half-lives.
NOTE: Diet, medications, and time of day may affect results. - Conduct the exam in a quiet, darkened room at 21 °C. When conducting longitudinal studies, hold repeat exams at the same time of day.
3. Baseline Measurements
- Ask the subject to lie supine on an exam table. Attach a 3-lead EKG in a standard position. Address any orthopedic issues to ensure the subject will be comfortable and refrain from movement during the exam.
- Allow the subject to rest for 10 min before the start of the exam. After 5 min rest, measure the subject's blood pressure by an oscillometric, non-invasive blood-pressure monitor.
- Apply a 5 cm tourniquet cuff in either a proximal or distal position to demonstrate the upper arm technique.
- Extend the subject’s arm laterally and maintain at the level of the heart.
- Depending upon the operator's preference, use a table and pillow to constrain the subject’s arm.
- Place the arm of the operator in a position that resists fatigue and provides support for the wrist. Try to minimize extension of the wrist and keep the forearm in the anatomic neutral position.
- Conduct a cross-sectional scan of the brachial artery, beginning at the insertion of the bicep and proceeding proximally. Use color flow imaging to verify the brachial artery and to locate collateral vessels that may serve as landmarks.
- When a suitable position is found, rotate the probe 90° so that the proximal edge appears on the left of the ultrasound screen. Maintain position on the artery using substantial practice and a delicate touch. Verify the orientation by pushing the tissue near the distal edge. Mark the subject’s skin along the distal edge of the probe.
- Align the focus setting of the probe with the deep or “far” wall of the brachial artery to improve lateral resolution of the image. Vary probe settings on the axial resolution with a higher frequency improving axial resolution.
- Adjust the angle of the probe to optimize contrast resolution of both the near and the far walls. Small changes to the angle can result in improved contrast. Estimate the angle with a simple protractor if serial exams are conducted on the subject.
- To ensure quality measurements, ensure that the vessel is horizontal and aligned with the longitudinal axis. Make small changes in pressure (heeling one edge of the probe) to help align the artery. Overall, keep pressure light to help prevent operator fatigue.
- When optimized, ensure that the “Double Lines of Pignoli” can be seen in both walls, corresponding to the intima-media thickness. Use gain adjustments to reduce echo in the vessel lumen. Allow at least 2 cm of intima-mediate thickness (IMT) on both sides for accurate diameter measurements.
4. Baseline Measurements
- Record the baseline velocity using 2D Doppler mode. Place the sample gates in the middle of the lumen and maintain an insonation angle of 60°. Collect 60 sec of data.
5. Occlusion Phase
- Inflate the cuff to 50 mm Hg above the subject’s systolic blood pressure. Using a 5 cm tourniquet cuff will overestimate the systolic pressure. Use the 2D Doppler mode to verify occlusion.
- Use a timer to track the duration of occlusion as many blood pressure cuffs will slowly lose pressure over 5 min. Use 2D Doppler mode to verify complete occlusion.
- After 4:30 of occlusion, place the 2D-Doppler gate slightly superficial to the longitudinal axis of the artery. Adjust the vertical scale to account for velocities 2-3x higher than baseline.
- Adjust the settings on the image capture software for 3:10 of recording.
- Begin recording 10 sec before cuff release to capture the time of cuff release, an important parameter when measuring time to peak diameter during data analysis.
6. Hyperemia
- Release the cuff. As the artery may shift superficially after cuff release, make small changes to the probe's position while listening for amplification of the sound to help compensate for the shift. Reposition the Doppler sample gate and insonation angle if the artery shifts.
- After 30 sec of velocity recording, switch the ultrasound to B-Mode.
- Since it is common for the probe to slide proximally during an exam, use vessel landmarks or the marking on the subjects' skin to verify probe position. This phase of the exam is critical for obtaining accurate results.
- Adjust the probe position or angle to optimize the IMT on both walls as small changes can substantially improve the image. Record the diameter for 3 min.
- If repeat measurements are planned, use the marking on the subject's skin to record distance from the antecubital fossa. Ask the subject to bend their arm 90° and mark the crease. Measure from this line to the line made earlier.
Representative Results
The key variables of flow-mediated vasodilation are shown in Table 1.
| Variable | Description |
| Mean Velocity (cm/sec) | The mean arterial velocity of blood in the middle 50% of the lumen during one cardiac cycle estimated from Doppler spectral waveforms, proportional to blood flow and inversely proportional to cross-sectional area (see Figure 1). |
| Diameter (mm) | The intima-intima distance as measured from a longitudinal view along the vessel axis (see Figure 2). This is measured at Baseline and during reactive hyperemia. |
| Flow (ml/min) | The bulk flow of fluid in the circulation, derived mathematically from mean velocity and diameter (see Equation 2). |
| Shear Stress (dynes/cm2) | The frictional force exerted by circulating blood on the intima surface, proportional to velocity and inversely proportional to diameter, derived from mean velocity and diameter (see Equation 3). |
| % FMD | The change in arterial diameter after occlusion in response to hyperemia, over the baseline diameter (see Equation 1). |
Table 1. The key variables of flow-mediated vasodilation.
This FMD protocol will provide sufficient data to measure % flow-mediated vasodilation, flow, and shear stress. Recording 60 sec of baseline data will help account for normal, physiological variation in heart rate and respiration. Analysis software will calculate diameter during the baseline and hyperemia phases. Some software packages can measure the average velocity (m/sec) during a point in the cardiac cycle by integrating the area under the velocity spectral waveform and dividing by time to arrive at a time-averaged velocity. Diameter and Velocity will allow an investigator to calculate the following variables.
Equation 1.
% FMD is defined as:  .
.
Equation 2.
Mean Flow in ml/min is defined as:  .
.
Equation 3.
Shear Stress is defined as:  where Tw is shear stress in dynes/cm2, Q is mean volumetric flow, and µ, the viscosity of blood, is assumed to be 0.035 poise.
where Tw is shear stress in dynes/cm2, Q is mean volumetric flow, and µ, the viscosity of blood, is assumed to be 0.035 poise.
Example data from studies conducted at the UCSF Vascular Integrated Physiology and Experimental Therapeutics lab (VIPERx) is given in Table 2 and Table 3. Briefly, the example cohort is a randomly chosen subset of participants in the cross-sectional arm of the Omega-PAD trial (NCT01310270)30. All participants were patients referred to the outpatient vascular surgery clinic of the San Francisco Veterans Affairs Medical Center for evaluation of peripheral artery disease (PAD). PAD diagnosis was based on current guidelines of an ankle-brachial index <0.9. Patients with incompressible arteries (ABI>1.4) were excluded. Inclusion in the “No PAD” group was based on ABI > 0.9 and the absence of PAD, CAD, and CVD. Statistical analysis was done by t-test for continuous variables or chi-square test for categorical variables.
The example cohort is almost entirely male with a mean age is 68 ± 9 years and Caucasian, 74%. As a whole, participants carry a number of cardiovascular risk factors including: hypertension (84%), hyperlipidemia (78%), smoking history (86%), and obesity (mean BMI is 30 ± 6). Overall, 16% of participants carry a diagnosis of coronary artery disease (CAD) and 40% a diagnosis of diabetes mellitus.
The prevalence of hypertension was higher in the PAD group than the non-PAD group (96% vs. 72%, p = 0.02) as was CAD (32% vs. 0%, p < 0.001) and diabetes (56% vs. 24%, p = 0.02). The PAD group had greater abdominal adiposity but not to the level of significance (waist-hip ratio of 1.04 vs. 1.00, p = 0.065). Likewise, the PAD group had worse low density lipoprotein (LDL) than the non-PAD group (68 vs. 101 mg/dl, p < 0.001) but better total cholesterol (142 vs. 174 mg/dl, p = 0.002). Both groups were appropriately managed with medications, demonstrating widespread use of statins and antihypertensive medications. The PAD group shows a higher level of aspirin (84% vs. 48%, p = 0.007) and beta-blockers (60% vs. 28%, p = 0.023), consistent with their co-morbidities.
Table 3 demonstrates example flow-mediated vasodilation data from the two groups. The baseline characteristics are similar for each group showing similar diameter, velocity, and flow. The PAD group, however, demonstrate worse flow-mediated vasodilation than the non-PAD group (6.8% vs. 9.1%, p = 0.021). Results for both group fall within the expected range for individuals with cardiovascular risk factors (<10%). A review of several studies suggest a %FMD of 6-10% in healthy adults and a %FMD of 0-5% in CAD populations using lower arm occlusion31-36. Values above 10% were observed in young, healthy adults using the upper arm technique37. The %FMD for each group has a wide standard deviation, presenting an opportunity to further segment the cohort based on %FMD.
Figures 1-4 demonstrate example images collected during the phases of FMD. Figure 1 shows a Doppler Spectral waveform obtained at Baseline. The arrows indicate the extent of one cardiac cycle which forms the basis for calculating mean arterial velocity. The protocol calls for averaging the results of multiple cycles collected during 60 sec. In the example cohort, the mean baseline velocity for all participants was 17 ± 6 cm/sec. No significant difference between the PAD and No PAD cohort was seen.
Figure 2 shows an example B-mode image of the Baseline vessel diameter. The arrows indicate the location where the intima-intima distance, the basis for the lumen diameter, was measured. In the example cohort, the mean baseline diameter for all participants was 4.20 ± 0.57 mm. No significant difference between the PAD and No PAD groups was seen.
Figure 3 shows an example Doppler Spectral waveform obtained immediately after cuff release in the Reactive Hyperemia phase. The yellow arrow indicates the moment of cuff release. Waveforms obtained in the first 5 sec after cuff are used to calculate the Reactive Hyperemia Velocity. For all participants, the mean Reactive Hyperemia Velocity was 74 ± 26 cm/s. No significant difference was seen between the PAD and No PAD groups.
Figure 4 shows example B-mode image obtained 60 sec after cuff release during the Reactive Hyperemia phase. Like the Baseline diameter, the intima-intima distance is used to calculate Reactive Hyperemia Diameter. For all participants, the mean Reactive Hyperemia diameter was 4.53 ± 0.59 mm. The difference between PAD and No PAD subgroups was approached, but did not meet, significance (p = 0.08). The difference between Baseline and Reactive Hyperemia diameter forms the basis of the numerator in the %FMD variable.
| Characteristics | All patients (n = 50) |
PAD (n = 25) |
No PAD (n = 25) |
P-value |
| Age, Mean (SD), y | 68 ± 9 | 68 ± 6 | 68 ± 11 | 0.89 |
| Male Sex (%) | 98 | 100 | 96 | 0.31 |
| Caucasian (%) | 74 | 84 | 64 | 0.37 |
| BMI | 30 ± 6 | 29 ± 7 | 30 ± 4 | 0.73 |
| Waist-hip ratio (%) | 1.02 ± 0.06 | 1.04 ± 0.06 | 1.00 ± 0.05 | 0.07 |
| Systolic Blood Pressure (mm Hg) | 136 ± 19 | 139 ± 22 | 134 ± 15 | 0.33 |
| Diastolic Blood Pressure (mm Hg) | 79 ± 10 | 78 ± 11 | 80 ± 10 | 0.47 |
| Index ABI | 0.93 ± 0.27 | 0.72 ± 0.16 | 1.14 ± 0.16 | <0.001 |
| Comorbidities | ||||
| Hypertension (%) | 84 | 96 | 72 | 0.02 |
| Hyperlipidemia (%) | 78 | 88 | 68 | 0.09 |
| Hx of CAD (%) | 16 | 32 | 0 | 0.00 |
| Diabetes Mellitus (%) | 40 | 56 | 24 | 0.02 |
| Medications | ||||
| Aspirin (%) | 66 | 84 | 48 | 0.01 |
| Ace-inhibitor (%) | 48 | 52 | 44 | 0.57 |
| β-Blocker (%) | 44 | 60 | 28 | 0.02 |
| Statin (%) | 66 | 68 | 64 | 0.77 |
| Insulin (%) | 30 | 14 | 6 | 0.39 |
| PAD Risk Factors | ||||
| History of smoking (%) | 86 | 92 | 79 | 0.24 |
| Total Cholesterol (mg/dl) | 158 ± 38 | 142 ± 31 | 174 ± 37 | 0.00 |
| LDL (mg/dl) | 85 ± 32 | 68 ± 27 | 101 ± 29 | <0.001 |
| HDL (mg/dl) | 44 ± 11 | 43 ± 11 | 46 ± 10 | 0.30 |
| Triglycerides (mg/dl) | 153 ± 119 | 165 ± 125 | 141 ± 115 | 0.49 |
| Hemoglobin A1C (%) | 6.3 ± 1.5 | 6.5 ± 1.5 | 6.1 ± 1.6 | 0.38 |
| Serum creatinine (mg/dl) | 1.11 ± 0.84 | 1.28 ± 1.15 | 0.95 ± 0.22 | 0.17 |
| eGFR (ml/min) | 80 ± 21 | 75 ± 21 | 86 ± 21 | 0.10 |
| Albumin (g/dl) | 4.0 ± 0.3 | 4.0 ± 0.3 | 4.1 ± 0.3 | 0.43 |
Table 2. Baseline characteristics of a sample cohort. The following data are a randomly chosen subset of participants in the cross-sectional arm of the Omega-PAD trial (NCT01310270) and cohort . All participants were patients referred to the outpatient vascular surgery clinic of the San Francisco Veterans Affairs Medical Center for evaluation of peripheral artery disease (PAD). PAD diagnosis was based on current guidelines of an ankle-brachial index <0.9. Patients with incompressible arteries (ABI > 1.4) were excluded. Inclusion in the “No PAD” group was based on ABI > 0.9 and the absence of PAD, CAD, and CVD.
| Characteristics | All patients (n = 50) |
PAD (n = 25) |
No PAD (n = 25) |
P-value |
| Baseline Artery Diameter (SD), mm | 4.20 ± 0.57 | 4.11 ± 0.60 | 4.29 ± 0.53 | 0.27 |
| Baseline Velocity (SD), cm/sec | 17 ± 6 | 18 ± 6 | 16 ± 5 | 0.13 |
| Baseline Flow (SD), ml/min | 145 ± 68 | 151 ± 84 | 138 ± 47 | 0.51 |
| Baseline Shear Stress (SD), dynes/cm2 | 12 ± 4 | 13 ± 5 | 11 ± 3 | 0.07 |
| Reactive Hyperemia Diameter (SD), mm | 4.53 ± 0.59 | 4.38 ± 0.60 | 4.68 ± 0.55 | 0.08 |
| Reactive Hyperemia Velocity (SD), cm/sec | 74 ± 26 | 70 ± 25 | 78 ± 27 | 0.32 |
| Reactive Hyperemia Flow (SD), ml/min | 735 ± 340 | 658 ± 327 | 812 ± 342 | 0.11 |
| Reactive Hyperemia Shear Stress (SD), dynes/cm2 | 46 ± 18 | 46 ± 19 | 47 ± 18 | 0.79 |
| Brachial FMD (%) | 8.0 ± 3.7 | 6.8 ± 3.5 | 9.1 ± 3.6 | 0.02 |
Table 3. Flow-Mediated Vasodilation analysis. As described in the protocol, baseline diameter and velocity are the mean of 60 sec of data. Reactive hyperemia diameter was obtained at 60 sec post-occlusion. Reactive hyperemia velocity was the time-averaged velocity of the first 5 sec of Doppler spectral waveforms obtained after cuff-release.
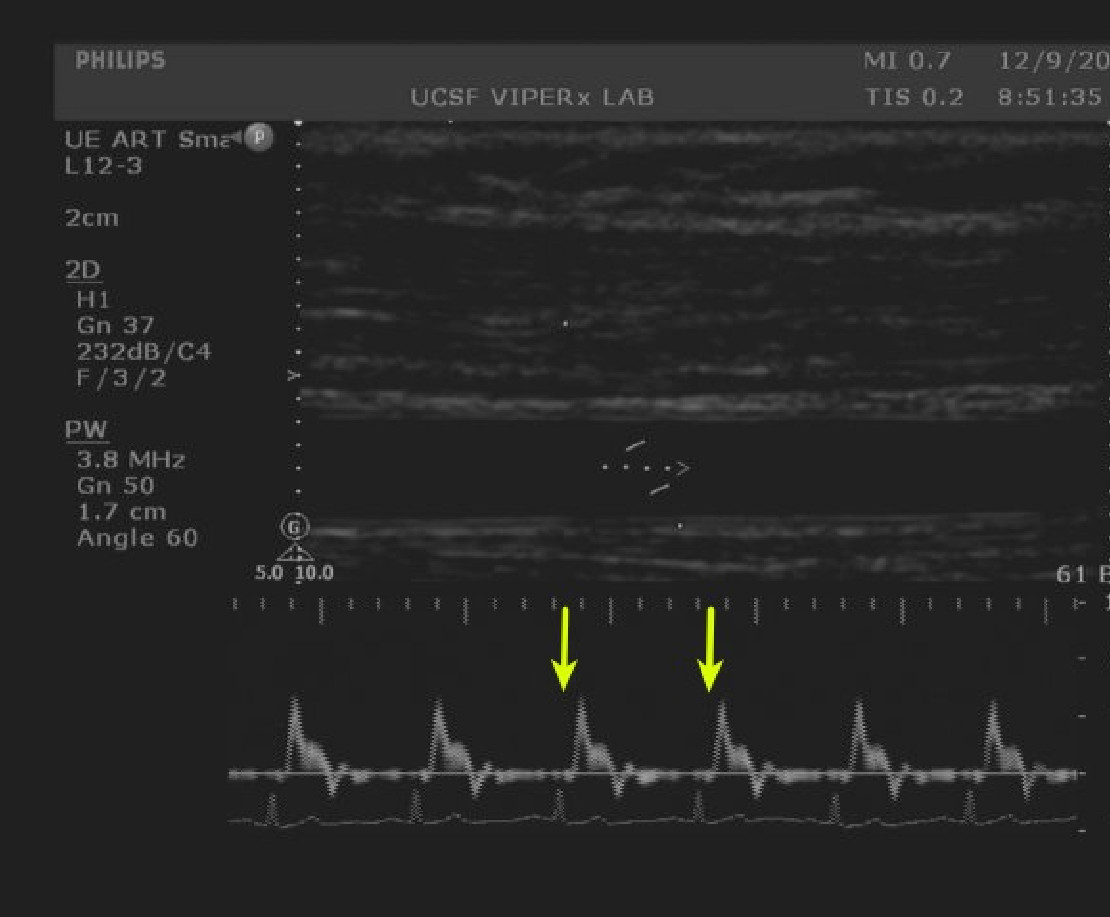
Figure 1. Baseline velocity measurements. Doppler spectral waveforms of the brachial artery are captured by an image analysis system. A single cardiac cycle is shown between the arrows. Image analysis systems can calculate the mean arterial velocity. Please click here to view a larger version of this figure.
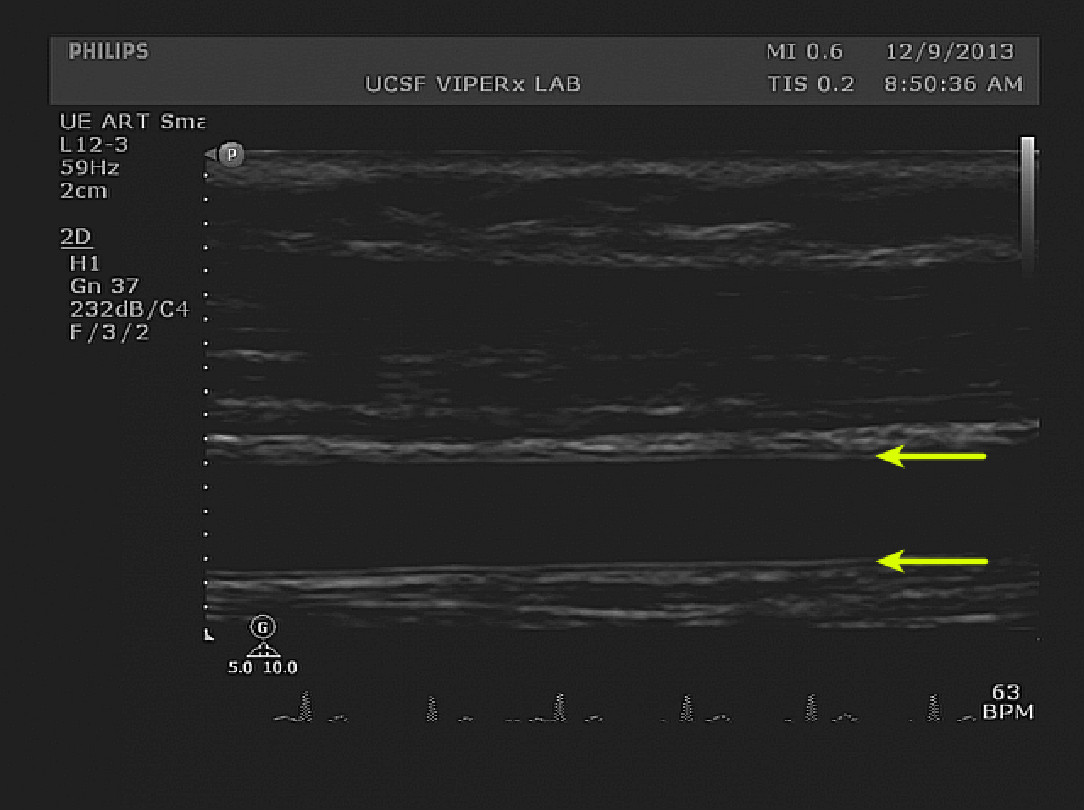
Figure 2. Baseline diameter measurements. The double-lines of Pignoli, corresponding to intima and media boundaries are visible on both the superficial and deep edges of the brachial artery (yellow arrows). The image shows proper horizontal and vertical alignment. Please click here to view a larger version of this figure.

Figure 3. Hyperemia velocity measurements. Doppler spectral waveforms immediately after cuff release are visible. The moment of cuff release can be appreciated by the sharp increase in velocity to the left of the image (yellow arrow). The top half of the image shows the positioning of the sample gate prior to cuff release. After cuff release, the artery may shift to a more superficial position. Placing the gate above the axis of the occluded artery helps compensate for vertical shifting of the artery after cuff release. Please click here to view a larger version of this figure.
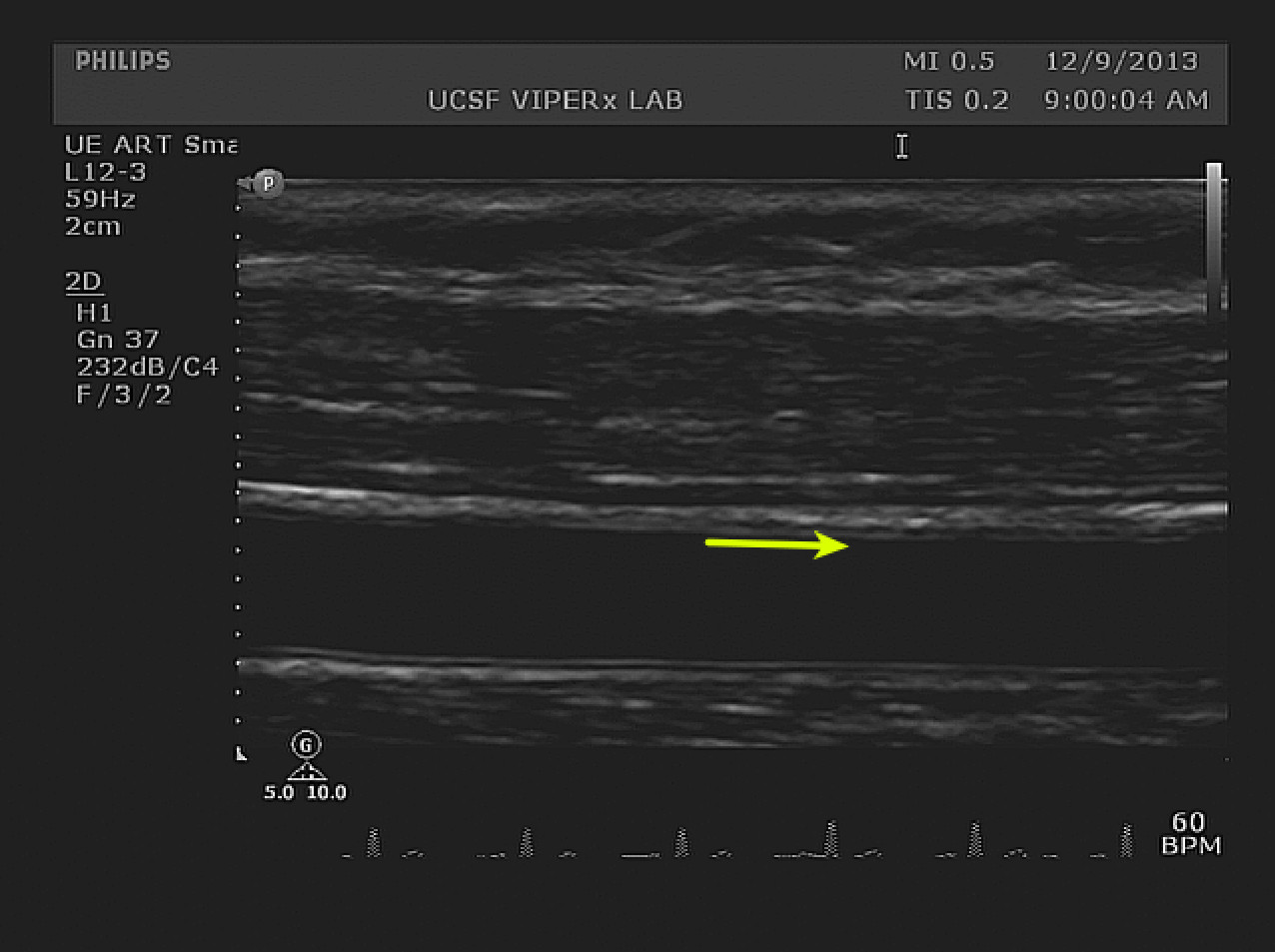
Figure 4. Hyperemia diameter measurements. A longitudinal view of the index segment after cuff release is visible. The change in diameter is small and may be quantified by image analysis software. The IMT boundary on the superficial wall is clearly visible along the index segment (yellow arrow). The width of the arrow shafts represents a 10% increase from the baseline diameter. Please click here to view a larger version of this figure.
Discussion
Endothelial dysfunction is an imbalance in the chemical mediators affecting vessel tone and an early step in the development of atherosclerosis. Measuring the reactivity of an artery is a way to assess the state of these chemical pathways. Both direct and indirect methods of assessing reactivity exist for the different vascular beds, ranging from direct infusion of an endothelium agonist in the coronary circulation to non-invasive, pulse waveform analysis in the index finger38.
Brachial artery FMD is an established technique for indirectly assessing endothelial function by high-frequency ultrasound. There are advantages to using FMD in clinical trials. First, the technique is non-invasive and easy to understand, which eases barriers to recruitment. Furthermore, use of invasive techniques, such as coronary angiography in asymptomatic patients raises ethical questions. Next, FMD requires a minimum amount of subject preparation compared to invasive techniques and the entire exam may be completed in a short amount of time. So long as the occlusion duration is kept within guidelines and subjects screened appropriately, FMD presents little concern for safety. The relative ease of an FMD study makes serial exams in longitudinal studies feasible, but its use in testing treatment effects is controversial. Likewise, according to current clinical guidelines, FMD is not appropriate for characterizing an individual’s risk for cardiovascular events or making clinical decisions39. The development of analysis software allows for quick analysis, blinding, and repeat analysis. Finally, technique is well established and a number of studies have been published using the technique, allowing for comparison of results40.
There are, however, challenges to the successful use of FMD in a trial. First, the technique presents a long learning curve. Current guidelines suggest a new technician complete 100 scans under an experienced operator before working independently. Next, the cost of equipment may be prohibitive for smaller laboratories. Subject preparation is important as factors, such as smoking, medications, post-prandial state, hyperglycemia, time-of-day, ambient temperature, and recent exercise can affect the magnitude of the response19,29. This requires careful instruction to the participant and rigorous adherence to study protocols. In older individuals, changes to vessel distensibility may diminish the predictive value of FMD19.
The protocol, as described above, measures %FMD at 60 sec post-occlusion. Studies have suggested peak FMD may occur outside of this window41,42. This protocol allows for capture of peak FMD by continuous recording for 3 min post-deflation. It should be noted this requires extensive experience and leads to longer analysis time. Likewise, the choice of upper-arm occlusion is controversial. In a study that compared different occlusion positions with similar shear stress stimuli, greater vasodilation was seen with the upper arm technique, suggesting some component of the dilation is not mediated by NO20. In a meta-analysis of studies using FMD, Bots et al. report a wide range of %FMD with the majority of the studies (81.2%) used the upper arm technique43. After adjusting for age, sex, presence of CHD, and diabetes, the lower arm technique was found to decrease %FMD (mean difference 2.47%, CI 0.55-4.39). While the location of the ischemic trigger (upper vs. lower arm) was found to be important, the location of the measurement (antecubital fossa vs. above antecubital fossa) was not significantly related to mean FMD. The difference in signal strength may be related to the size of the ischemic bed. Current guidelines support the use of either the upper arm or lower arm technique, suggesting laboratories adopt a consistent method across trials.
There are a number of critical steps in FMD. First, subject preparation is paramount as a number of factors such as medications, diet, nicotine, and exercise may affect the participant’s response. Sympathetic activation reduces FMD, so take appropriate steps to minimize distraction or discomfort for the participant44. Next, selecting an appropriate index segment of the brachial artery will improve the accuracy of the test. Distinct intima lines are important for measuring the change in diameter after occlusion. The change in diameter is small, typically 5-10% of a 5 mm artery or 50-100 μm, and the diameter of the artery varies longitudinally. Operator fatigue is common and the ultrasound probe may slide during the exam to a section of the vessel with a different diameter. It is important to have visually distinct landmarks such as collateral vessels or areas of medial calcification to verify the index segment. Use of a stereotactic clamp to hold the probe or simply marking the subject’s skin can help maintain the index segment. Likewise, steps to prevent the subject from unintentionally moving their arm, such as shoulder and forearm constraining pillows, are recommended.
If holding the probe by hand, care should be taken to prevent operator fatigue or repetitive use injury. We suggest arranging the participant and any equipment so that the operator’s forearm is in the anatomic neutral position. Minimize the force required to hold the probe by wrapping it in foam tape or use a vertical post to clamp the cable, reducing the force required to hold the probe in a static position for 10 min.
Measuring velocity and diameter in the hyperemia presents technical challenges. This protocol calls for measuring 30 sec of Doppler spectral waveforms to record velocity then switching to B-mode imaging for diameter measurements. The peak velocity will occur between 5-10 sec after cuff release and the vessel may shift position during this time. If the probe is off the longitudinal axis or the insonation angle is >60°, the magnitude of the waveforms will be inaccurate. Positioning the sample gate slightly above the longitudinal axis of the vessel will help account for any shifting. When switching from Doppler to B-mode, it is important to maintain a position over the index segment. It is likely the artery has shifted and there is only a short time-window (25 sec) to optimize the image for the hyperemia diameter measurement. Often, only small changes to probe pressure, longitudinal alignment and angle are necessary to produce a quality image of the index segment. If the vessel is completely lost, a quick transverse scan up the artery will allow identification of the index segment, the most echogenic portion of the intima, and the correct angle of approach. Then, rotating the probe to a longitudinal view will return to a suitable view of the index segment.
Simple steps can improve the quality and consistency of FMD studies. The UCSF Vascular Integrated Physiology and Experimental Therapeutics (VIPERx) lab uses a quality control protocol when conducting FMD studies. First, participants are given standard instructions and pre-visit phone calls to ensure they avoid medications and behavior that affect the study. Next, a standard set of flow-sheets are used by study staff to ensure the exam is conducted the same way for each participant and critical steps are not overlooked. Finally, a post-exam six-point grading system is used to rate each study. Important factors such as the alignment of the vessel relative to the probe, the presence of anatomical landmarks, and the extent of intima lines are included to ensure these critical steps are met.
Disclosures
The authors have nothing to disclose.
Acknowledgements
From the Vascular Integrated Physiology and Experimental Therapeutics (VIPERx) Laboratory, this work was supported by funds from the Department of Surgery, University of California, San Francisco and the Northern California Institute for Research and Education. The project described was supported by Award Number KL2RR024130 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Philips HD 11XE ultrasound | Philips Healthcare | ||
| 5-12 MHz linear array transducer | Philips Healthcare | L12-5 | |
| Ultrasound gel | Parker Laboratories | ||
| Vascular Research Tools v.5.0 | Medical Imaging Applications, LLC | ||
| MIA Gating module | Medical Imaging Applications, LLC | ||
| Desktop PC | Dell, Inc | ||
| Windows XP | Microsoft, Inc | ||
| 5 cm tourniquet blood pressure cuff | Hokanson | SC 5 | |
| Hand-held aneroid manometer | Welch Allyn | DS66 |
References
- Gerlach, E., Nees, S., Becker, B. F. The vascular endothelium: a survey of some newly evolving biochemical and physiological features. Basic Res Cardiol. 80, 459-474 (1985).
- Sato, T., Arai, K., Ishiharajima, S., Asano, G. Role of glycosaminoglycan and fibronectin in endothelial cell growth. Experimental and molecular pathology. 47, 202-210 (1987).
- Yanagisawa, M., et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 332, 411-415 (1988).
- Ignarro, L. J., Buga, G. M., Wood, K. S., Byrns, R. E., Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences. 84, 9265-9269 (1987).
- Moncada, S., Higgs, E. A., Vane, J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. The Lancet. 309, 18-21 (1977).
- Ozkor, M. A., et al. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 123, 2244-2253 (2011).
- Suwaidi, J. A., et al. Long-Term Follow-Up of Patients With Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation. 101, 948-954 (2000).
- Neunteufl, T., et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 129, 111-118 (1997).
- Taddei, S., et al. Hypertension Causes Premature Aging of Endothelial Function in Humans. Hypertension. 29, 736-743 (1997).
- Perticone, F., et al. Prognostic Significance of Endothelial Dysfunction in Hypertensive Patients. Circulation. 104, 191-196 (2001).
- Williams, S. B., Cusco, J. A., Roddy, M. -. A., Johnstone, M. T., Creager, M. A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. Journal of the American College of Cardiology. 27, 567-574 (1996).
- Schindler, T. H., et al. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol. 23, 495-501 (2003).
- Halcox, J. P., et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 106, 653-658 (2002).
- Yeboah, J., et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study the multi-ethnic study of atherosclerosis. Circulation. 120, 502-509 (2009).
- Ludmer, P. L., et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. New England Journal of Medicine. 315, 1046-1051 (1986).
- Higashi, Y., et al. Effect of the angiotensin-converting enzyme inhibitor imidapril on reactive hyperemia in patients with essential hypertension: relationship between treatment periods and resistance artery endothelial function. Journal of the American College of Cardiology. 37, 863-870 (2001).
- Linke, A., Erbs, S., Hambrecht, R. Exercise and the coronary circulation—alterations and adaptations in coronary artery disease. Progress in cardiovascular diseases. 48, 270-284 (2006).
- Celermajer, D. S., et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. The Lancet. 340, 1111-1115 (1992).
- Thijssen, D. H. J., et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American Journal of Physiology – Heart and Circulatory Physiology. 300, (2011).
- Doshi, S. N., et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clinical science. 101, 629-635 (2001).
- Brevetti, G., Silvestro, A., Schiano, V., Chiariello, M. Endothelial Dysfunction and Cardiovascular Risk Prediction in Peripheral Arterial Disease: Additive Value of Flow-Mediated Dilation to Ankle-Brachial Pressure Index. Circulation. 108, 2093-2098 (2003).
- Neunteufl, T., et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. The American Journal of Cardiology. 86, 207-210 (2000).
- Gokce, N., et al. Predictive value of noninvasivelydetermined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. Journal of the American College of Cardiology. 41, 1769-1775 (2003).
- Gokce, N., et al. Risk Stratification for Postoperative Cardiovascular Events via Noninvasive Assessment of Endothelial Function: A Prospective Study. Circulation. 105, 1567-1572 (2002).
- Anderson, T. J., et al. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 26, 1235-1241 (1995).
- De Roos, N. M., Bots, M. L., Schouten, E. G., Katan, M. B. Within-subject variability of flow-mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound in medicin., & biology. 29, 401-406 (2003).
- Berry, K. L., Skyrme-Jones, R. A., Meredith, I. T. Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery flow-mediated dilation. Clinical science. 99, 261-267 (2000).
- Betik, A. C., Luckham, V. B., Hughson, R. L. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. American journal of physiology. Heart and circulatory physiology. 286, 442-448 (2004).
- Corretti, M. C., et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial arteryA report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 39 (1001), 257-265 (2002).
- Grenon, S. M., et al. n-3 Polyunsaturated fatty acids supplementation in peripheral artery disease: the OMEGA-PAD trial. Vascular medicine. 18, 263-274 (2013).
- Moens, A. L., Goovaerts, I., Claeys, M. J., Vrints, C. J. Flow-mediated vasodilation. Chest. 127, 2254-2263 (2005).
- Gnasso, A., et al. Association between wall shear stress and flow-mediated vasodilation in healthy men. Atherosclerosis. 156, 171-176 (2001).
- Verma, S., et al. Cross-sectional evaluation of brachial artery flow-mediated vasodilation and C-reactive protein in healthy individuals. European Heart Journal. 25, 1754-1760 (2004).
- Donald, A. E., et al. Methodological Approaches to Optimize Reproducibility and Power in Clinical Studies of Flow-Mediated Dilation. Journal of the American College of Cardiology. 51, 1959-1964 (2008).
- Witte, D. R., et al. Is the Association Between Flow-Mediated Dilation and Cardiovascular Risk Limited to Low-Risk Populations. Journal of the American College of Cardiology. 45, 1987-1993 (2005).
- Benjamin, E. J., et al. Clinical Correlates and Heritability of Flow-Mediated Dilation in the Community: The Framingham Heart Study. Circulation. 109, 613-619 (2004).
- Nosova, E. V., et al. Short-term Physical Inactivity Impairs Vascular Function. Journal of Surgical Research. 10, (2014).
- Axtell, A. L., Gomari, F. A., Cooke, J. P. Assessing Endothelial Vasodilator Function with the Endo-PAT. Journal of Visualized Experiments. , (2000).
- Greenland, P., et al. ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic AdultsA Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Journal of the American College of Cardiology. 56, (2010).
- Inaba, Y., Chen, J. A., Bergmann, S. R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. The international journal of cardiovascular imaging. 26, 631-640 (2010).
- Black, M. A., Cable, N. T., Thijssen, D. H. J., Green, D. J. Importance of Measuring the Time Course of Flow-Mediated Dilatation in Humans. Hypertension. 51, 203-210 (2008).
- Chironi, G., Craiem, D., Miranda-Lacet, J., Levenson, J., Simon, A. Impact of shear stimulus, risk factor burden and early atherosclerosis on the time-course of brachial artery flow-mediated vasodilation. Journal of Hypertension. 26, 508-515 (2008).
- Bots, M. L., Westerink, J., Rabelink, T. J., Pd Koning, E. J. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. European Heart Journal. 26, 363-368 (2005).
- Hijmering, M. L., et al. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. Journal of the American College of Cardiology. 39, 683-688 (2002).

