液-液抽出の効率
English
Share
Overview
ソース: ケリー ・ m ・ ドゥーリーとマイケル g. ベントン、工業化学科、ルイジアナ州立大学、バトン ルージュ, ルイジアナ
液-液抽出 (LLE) は蒸留の代わりに用いる分離技術とどちらか: (a) 分離する化合物の相対的なボラティリティが非常に似ています。(b) 1 つ以上の混合物成分の環境状態にも近い敏感な温度は、します。(c) 蒸留には、非常に低圧または非常に高い留出油/飼料の比率を必要があります。1物質移動の原動力は、2 つ他不混和性または部分的に水溶性のストリーム (フィードと溶媒) の 1 つのマテリアル (溶質) の溶解度の違いです。フィードおよび溶媒のストリームの混合し、分離し、フィードから溶媒に転送する溶質を許可します。通常、対向流を用いた連続した段階でこのプロセスが繰り返されます。溶質の豊富な溶媒は放っておいて、溶質枯渇フィードはエマルションと抽出と呼ばれます。フィードと溶媒のストリームとの間の合理的な密度差が抽出できます縦の列を使用して一連の混合と図り、沈降槽その他の場合使用できます。
この実験ではイソプロパノール (IPA, 10 〜 15 重量 %、溶質) を抽出することです C8の混合物から運用の目標-に-C10炭化水素溶媒として純水を使用します。ニューヨーク Scheibel 型 (垂直ミキサーと coalescers、物理的な段階ごとに 1 つ) 抽出列が使用。ほとんどの抽出のようなこの列の全体的な効率 (数理論的な段階の物理的な段階) が非常に低く、特に多くの蒸留塔と比較しています。低効率にもフェーズの偏在から両方遅い大量転送 (蒸留のような 1 つではなく 2 つの液体抵抗) から、多くの場合が発生します。抽出と全体的なカラム効率両方溶質回収撹拌速度の影響が評価されます。
Principles
Procedure
Results
Figures 3 and 4 show results when both the agitation and feed flow rates were varied over a wide range. The overall efficiency and recovery increase before becoming asymptotic, which is fairly typical of liquid-liquid extractors that are not at or near flooding. At near flooding conditions, the overall efficiency and recovery are expected to sharply decrease. Note that, unlike distillation, flooding can take place in liquid-liquid extraction at either high solvent or high feed rates (or ratios).1 In this experiment, the lighter organic phase is also the dispersed (droplet) phase, so at high feed rates it is expected that the droplets coalescence prior to flooding, leading to lower rates of mass transfer and, therefore, lower recoveries and efficiencies. At high solvent rates the droplets should remain small, so it is expected that the recovery and efficiency remain high until very near the flooding point.
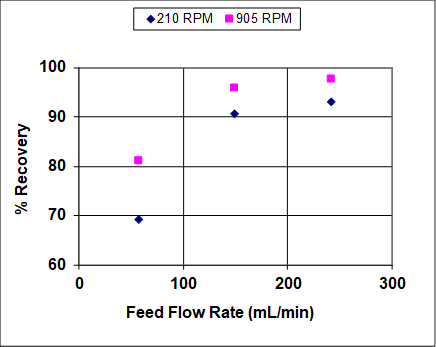
Figure 3: Percent recovery of IPA from hydrocarbon mixture into water, for a York-Scheibel column, 11 stages, 16 – 18 mol% IPA in Isopar E (feed), S/F (molar) = 1.5.
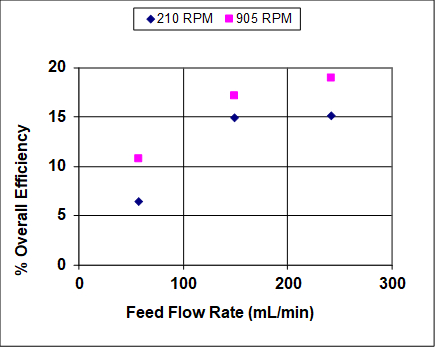
Figure 4: Percent overall stage efficiency for IPA extraction using a York-Scheibel column, 11 stages, 16 – 18 mol% IPA in hydrocarbon mixture (feed), S/F (molar) = 1.5.
As seen from Figures 3 and 4, increasing the agitation rate increases both the recovery and overall efficiency. This is because with greater power input the droplets of the dispersed phase are smaller – the observed dependence is roughly inverse with respect to agitator speed.4 The "a" parameter (interfacial area/total volume) that appears in mass transfer correlations and fundamental mass flux equations can be written as follows for uniform-size spherical droplets:
a = 6 ε/d (4)
where ε is the volume fraction of the dispersed phase. While ε can increase with an increase in either phase's superficial velocity, its changes are usually less marked than the change of diameter with respect to speed. So (usually) the more speed, the more interfacial area, leading to faster mass transfer.
The exception to the above discussion is at very high speeds, which were not reached in Figs. 3 and 4, where the two phases are so well-mixed that if the interfacial tension between them is low, emulsification will take place. The formation of an emulsion negatively impacts recovery and efficiency because the phases can no longer separate cleanly to move up or down to the next physical stage.Emulsification is a problem in many liquid-liquid extractions and where it cannot be limited a series of mixer and settler vessels in series is often preferred to column-type designs such as sieve trays or York-Scheibel units.
Applications and Summary
Liquid-liquid extraction (LLE) is an alternative to distillation which relies upon solvent-feed immiscibility (or slight miscibility) and favorable solute partition coefficients to attain high solute recoveries in a solvent phase at as low a solvent/feed ratio as practical. Although the range of flows (the "turndown") over which LLE will be effective is often limited, and while stage efficiencies are low such that phase equilibrium is not attained, certain mixtures just cannot be separated using other methods in a continuous countercurrent process. Mathematical analysis of the equilibrium operation of such extractors follows a familiar McCabe-Thiele-type procedure (although reflux is often lacking, so only one operating line). The non-equilibrium ("rate-based") analysis of LLEs is complex and depends strongly on the relative velocity between the two phases (the "slip velocity"), bubble size, and dispersed phase fraction, all of which can be observed but are difficult to predict.
To perfectly describe the hydraulics and mass transfer of a typical LLE is beyond the capability of even the most sophisticated process simulators, at present. Therefore, design of industrial units still relies on scale-up from pilot-plant-type units, such as that which was tested in this experiment. Normally the engineer attempts to duplicate key descriptors such as the "a" parameter, solvent/feed ratio, total agitator power input/volume, feed location and number of physical stages to keep the stage efficiency and recovery constant during scale-up. Even so, scale-up is an inexact science, and impurities, which alter the interfacial tension, can greatly impact the performance of real systems. The more factors that are held constant, the more likely the scale-up will be successful.
There are many different LLE contactors: a series of mixers – settler vessels, structured packings similar to those used in absorbers and distillation columns, sieve tray columns, rotating disk contactors (similar to the York-Scheibel, but with baffles instead of mesh), Kuhni contactors (a combination of rotating disk and sieve trays), and Podbielniak contactors ("Pods"), where the flow is radial and centrifugal force is used to enhance liquid phase separation.5
A classic example of industrial LLE is the separation of acetic acid from water using ethyl ether or ethyl acetate;6 it is preferred over distillation at lower acetic acid concentrations. Possibly the biggest volume LLE process is that of propane deasphalting, which is used to refine lubricating oils in refineries at near-supercritical conditions.1 However, most applications are found in the production of specialty chemicals and in pharmaceutical industries, ranging from citric acid extraction from fermentation broth to purification of antibiotics and protein purifications.1In these cases, a wide variety of oxygenated organic solvents or two-phase aqueous systems (with one phase being mostly water and the other aqueous dissolved salts and polymers) are utilized. For the latter, a typical polymer system is poly(ethylene glycol)/dextran with NaCl and Na2SO4 as salts. Applications include red blood cell separation and extraction of the phophofructokinase enzyme from S. cerevisiae.7
Appendix – Equilibrium Data8
Experimental Tie Lines in Mole Percent at 25 °C

Specific Model Parameters in Kelvin
| UNIQUAC | NRTL (a = 0.2) | ||||
| I | J | AIJ | AJI | AIJ | AJI |
| 1 | 2 | -186.05 | 104.6 | 814.26 | -468.11 |
| 1 | 3 | 361.91 | 621.82 | 3151 | 1367.4 |
| 2 | 3 | -126.43 | 311.7 | 581.79 | -25.91 |
R1 = 0.92 R2 = 2.7792 R3 = 6.523
Q1 = 1.4 Q2 = 2.508 Q3 = 5.476
| Mean Deviation between Calculate and Experimental Concentrations in Mol. % | |
| UNIQUAC (specific parameters) | 1.4 |
| NRTL (specific parameters) | 0.54 |
| UNIQUAC (default parameters) | 1.68 |
References
- T.C. Frank, L. Dahuron, B.S. Holden, W.D. Prince, A.F. Seibert and L.C. Wilson, Ch. 15 of “Chemical Engineers Handbook, 8th Edition”, R.H. Perry and D.W. Green, Eds., McGraw-Hill, New York, 2008.
- W.L. McCabe, J.C. Smith, and P. Harriott, “Unit Operations of Chemical Engineering”, 7th Ed., McGraw-Hill, New York, 2005, Ch. 23; C.J. Geankoplis, “Transport Processes and Unit Operations”, 3rd Ed., Prentice-Hall, Englewood Cliffs, 1993, Ch. 12; R.K. Sinnott, “Coulson and Richardson’s Chemical Engineering Vol. 6 – Chemical Engineering Design (4th ed.): http://app.knovel.com/hotlink/toc/id:kpCRCEVCE2/coulson-richardsons-chemical/coulson-richardsons-chemical
- B.E. Poling, G.H. Thomson, D.G. Friend, R.L. Rowley and W.V. Wilding, Ch.2 of “Chemical Engineers Handbook, 8th Edition”, R.H. Perry and D.W. Green, Eds., McGraw-Hill, New York, 2008.
- J.C. Godfrey, R. Reeve and F.I.N. Obi, Chem. Eng. Prog. Dec. 1989. pp. 61-69; I. Alatiqi, G. Aly, F. Mjalli and C.J. Mumford, Canad. J. Chem. Eng., 73, 523-533 (1995).
- http://www.pharmaceuticalonline.com/doc/podbielniak-contactor-a-unique-liquid-liquid-0003 (accessed 12/19/16).
- C.J. King, Ch. 18.5 of “Handbook of Solvent Extraction”, T.C. Lo, M.H.I Baird and C. Hanson, Eds., Wiley, New York, 1983.
- “Methods in Enzymology, Vol. 228, Aqueous Two-Phase Systems,” H. Walter and G. Johannson, Eds., Academic, San Diego, 1994.
- A.I. Vorobeva and M.Kh. Karapetyants, Zh. Fiz. Khim., 41, 1984 (1967). Fits to data from: J. Gmehling, and U. Onken, "Vapor-liquid equilibrium data collection", Dechema, 1977.
Transcript
Liquid-liquid extraction, or LLE, is a technique used to separate liquids that can not be separated with distillation due to temperature-sensitive components or similar solvent boiling points. Mass transfer in LLE is driven by the solubility difference of the solute in the immiscible or partially miscible feed and solvent streams. The feed stream containing the solute is mixed with the solvent stream, often using an agitator, allowing the solute to transfer from the feed to the solvent. The depleted feed, known as the raffinate, is separated from the extract, which is the solute-rich solvent phase. This video will illustrate an extraction of isopropanol from n-nonane, using pure water and study how operating variables affect the overall column efficiency.
LLE is typically performed in continuous stages using co-current or counter-current flow. Counter-current systems are generally preferred, as they tend to be more efficient. Usually, the stages are housed within a single unit. Counter-current extraction columns can be set up two ways. When the solvent is heavier than the feed liquid, or diluent, the solvent is introduced at the top of the column and the solute then exits at the bottom. When the solvent is lighter than the diluent, the solvent is introduced at the bottom of the column, and the solute will exit the column at the top. At steady-state, the material balance of the solute between the feed-end of the process and any stage denoted by N is as shown. Where X is the mole fraction of solute in the diluent, Y is the mole fraction of solute to the solvent. F is the molar flow-rate of feed diluent and S is the molar flow-rate of solvent. The analysis of theoretical plates is used to evaluate the efficiency of the separation process. These plates are hypothetical stages where two phases are in equilibrium with each other. If the two liquids are in equilibrium at a stage, meaning that there would be no change in concentration of either given longer mixing time, then the stage is considered to be a theoretical plate. The higher the number of theoretical plates, the more efficient the process. Operating variables such as temperature, pressure, flow rates and agitator speed affect efficiency and therefore theoretical plate analysis. The following experiment will examine an LLE process to extract the solute isopropanol from n-nonane using water as the solvent. This system, containing three liquids, is called a ternary system. Often, all three liquid components are miscible to some degree. Equilibrium behavior for these and other solvents can be found in the literature. Now that the basics of LLE and its operation have been explained, let’s take a look at a separation process.
A York-Scheibel column will be used for this experiment. It is an agitated column with internal paddle-wheel impellers, connected to one vertical drive. Each level contains wire-mesh packing to enable phase-separation, and is separated by partitions to provide individual stages. First use the control knob and digital readout on the control panel to control the speed of the agitator. Use the rotameters on the feed and solvent inlets to measure the flow rate of feed and solvent. Use graduated cylinders and a watch to measure the flow rates of the extract and raffinate. Now start the mixer and keep agitator speed constant at 300 rpm. Open the ball valves for the solvent, feed, extract and raffinate. Start the flow of water into the column to obtain the desired solvent-to-feed molar ratio at a rate of 200 milliliters per minute. Observe whether an interface between solvent entrance and raffinate exit is present, and if not, let the dispersed phase rise to form the upper interface. Start the feed flow when the upper interface forms. Carefully adjust the height of the inverted U on the extract line from the bottom of the tower, to control the level of the upper interface between the two phases. This assures that the raffinate phase does not flow into the extract tank if adjusted too low, or that the extract is not flowing into the raffinate tank if interface is set too high.
Collect samples every 10 minutes at the raffinate sample point in four milliliter bottles. Use gas chromatography, or GC, to quantify the components and confirm that a steady-state has been reached. Next, using a clean graduated cylinder, collect 250 milliliters of the extract at the sample point, then measure the specific gravity or relative density of the sample to water, using a hydrometer. Interpolate the weight per cent of the isopropanol in the sample using the provided table, which displays extract stream composition versus specific gravity. Repeat the procedure for two other lower feed-rates. Make sure to keep both the solvent-to-feed ratio and the agitator speed constant. When finished, turn off the agitator and main power switch and close the feed and solvent ball valves, leaving the raffinate and extract ball valves open. Now let’s evaluate the results.
First, let’s take a look at the per cent recovery of isopropanol with varied feed-flow rate and varied agitation rate. With increased feed-flow rate, per cent recovery increases and levels off. This is typical of a system which is not near flooding. Increased agitation rate also increased per cent recovery. Stage efficiency, calculated using theoretical plate analysis via computer simulation is also affected by these parameters. As expected, both stage efficiency and per cent recovery increase with higher flow-rate and agitation. This is due to improved mixing, which results in smaller droplets and improved dispersion, thereby improving mass transfer, however, both relationships plateau at higher feed rates. Efficiency and per cent recovery level off and eventually decrease due to emulsification and flooding. The formation of an emulsion negatively impacts recovery and efficiency because the phases can no longer separate cleanly in order to move up or down to the next stage. This can be a problem in systems like the York-Scheibel unit, making mixer and settler vessels in series an appealing alternative.
Liquid-liquid extraction is a separation technique used in a wide range of separations and can be used in a variety of setups. In the case where the emulsification of phases is a challenge, mixer settler tanks in series can be used. This simple setup utilizes a tank where the two phases are mixed by an agitator. The two phases then coalesce in the settler tank, where the heavy phase eventually settles to the bottom and is removed through an outlet on the bottom of the tank. The light phase settles to the top and is removed via another outlet. Another separation technique that harnesses the solubility properties of a solute is solid-liquid extraction. In solid-liquid extraction, the solute present in a solid matrix is extracted into a liquid through vigorous mixing. This technique is used on a large scale for many applications, such as the removal of toxins like the herbicide Atrazine from soil.
You’ve just watched Jove’s introduction to liquid-liquid extraction. You should now understand the basics of an LLE extraction using a York-Scheibel column and how process variables such as agitator and flow-rate can affect the solute recovery and efficiency of the column. Thanks for watching.
