Purificação do ferroceno por sublimação
English
Share
Overview
Fonte: Tamara M. Powers, Departamento de Química da Texas A&M University
A sublimação, a transição de fase direta de um sólido para um gás sem antes se tornar um líquido, ocorre a temperaturas e pressões inferiores às do ponto triplo do composto(Figura 1). O processo de sublimação pode ser utilizado para purificar sólidos orgânicos e inorgânicos. Durante a técnica de purificação, um sólido é aquecido diretamente na fase gasosa. Todas as impurezas não voláteis são deixadas para trás enquanto o composto vaporizado é então coletado (deposição) como um sólido em uma superfície fria. Aqui, usaremos sublimação para purificar ferroceno, um sólido inorgânico com uma temperatura de ponto triplo de 183 °C.1
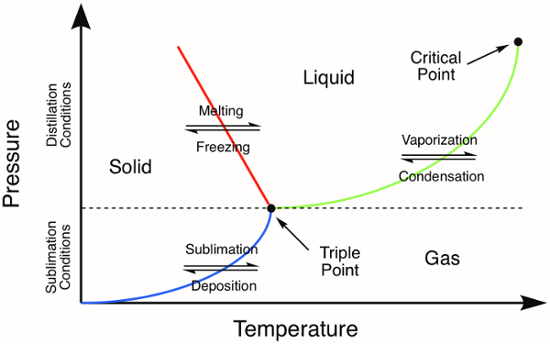
Figura 1. Diagrama de fase genérico. As linhas coloridas representam os requisitos de pressão e temperatura para transições de fase. A destilação de um sólido ocorrerá em pressões e temperaturas acima do ponto triplo, representado pela linha verde no diagrama de fase. A linha azul representa as condições de temperatura e pressão onde ocorre a sublimação.
Principles
Procedure
Results
Ferrocene (99%) was purchased from Alfa Aesar. Sublimation of 500 mg as described resulted in 493 mg isolated product. The purified ferrocene was analyzed by 1H NMR. 1H NMR (chloroform-d, 300 MHz, δ, ppm): 4.17 (s).
Applications and Summary
Sublimation is a technique used in the purification of solids. Solids that sublime at low pressure and temperature are good candidates for purification by sublimation. Here, we have demonstrated how to use a sublimation chamber to sublime ferrocene under static vacuum at 80 °C.
In a laboratory setting, sublimation is a useful technique that can be applied to the purification of solids in a variety of situations including in the purification of starting materials or synthesized products. In this example, the purified solid is collected on the cold finger, while the impurities are left at the bottom of the sublimation chamber. However, one may want to remove a solid impurity that can be sublimed from a non-volatile solid. In this case, the desired material remains at the bottom of the sublimation chamber.
Sublimation is also used in freeze-drying, called lyophilization. Lyophilization is a process to dry materials used in both pharmaceutical and food industries, as well as in research laboratories. In the lyophilization process, a material is first frozen, followed by reduction of the surrounding pressure, which allows the water (or other solvent) to be removed by sublimation.
References
- Kaplan, L., Kester, W. L., Katz, J. J. Some properties of iron biscyclopentadienyl. J Am Chem Soc. 74, 5531-5532 (1952).
Transcript
Sublimation is the phase transition of a substance from solid into gas without passing through its intermediate liquid phase. It is an important technique used for purification of organic and inorganic solids.
Usually the transition from solid to gaseous state requires passing through its liquid state.
However, reduced pressure and heating of a solid can lead to volatilization without melting, known as sublimation. The reverse process in which the substance passes from its gaseous to its solid state, is called deposition.
This video will illustrate the principles of sublimation, a typical procedure, and several applications.
At normal pressures, most chemical compounds and elements possess three different states of matter at different temperatures with a triple point at which all three states are present.
As seen in a phase diagram, vaporization and condensation – together known as distillation – may be performed at pressures above the compound’s triple point.
On the contrary, sublimation and deposition occur only at pressures that lie below the triple point.
Sublimation can be performed using two types of apparatus, depending on the volatility of the solid: for highly volatile compounds, a makeshift sublimation chamber may be assembled from a beaker and a watch glass. This method is appropriate for compounds that sublime at or near atmospheric pressure and ambient temperature.
If vacuum and/or inert atmosphere are required, a specialized piece of glassware made specifically for sublimation is used. It is made of a glass cup, containing the crude solid, and a hollow cylinder, which contains a cryogen and fits over the top of the cup. An O-ring seals the base and cold finger, and a vacuum attachment makes up the rest of the apparatus.
After completing the sublimation procedure, the apparatus is disassembled in a fume hood or glovebox depending on whether the material is air-sensitive. Then the purified solid may be scraped off of the cylinder, while the non-volatile impurities remain in the cup.
Now that we have discussed the principles of sublimation, let’s take a look at an actual procedure.
In a fume hood equipped with a Schlenk line, or dual manifold, weigh 500 mg of ferrocene in the base of a sublimation chamber.
Place an O-ring in the groove of the chamber base, and gently place the cold finger into the chamber base, making sure the O-ring fits. Then secure the two pieces of the chamber with a clamp.
Connect the assembled chamber to the Schlenk line, and open the chamber to vacuum for 1 min. Then close the vacuum valve on the chamber to continue the experiment under static vacuum.
Clamp the chamber to a ring stand and place the base portion of the chamber in an 80 °C bath. Fill the cold finger with an ice-slurry, replenishing it as it warms.
After sublimation is complete, remove the chamber from the bath. Close the stopcock to the Schlenk line and detach the tube from the chamber.
Then, repressurize the chamber by slowly opening the valve to air in a fume hood or glovebox.
Use a pipette to remove water from the cold finger and unclamp the two pieces of the chamber. Then carefully lift the cold finger out of the sublimation chamber.
Scrape the purified ferrocene from the cold finger with a spatula, transfer to a pre-weighed vial, and record the weight.
500 mg of purchased ferrocene was purified via sublimation resulting in 493 mg isolated product with a yield of 99.6%. The proton NMR shows a singlet at 4.17 ppm, which integrates to 10 protons of the ferrocene. The absence of other peaks indicates that no impurities are present, and that the purification was successful.
Now that we have discussed a procedure for sublimation, let’s take a look at a few applications.
Water can be sublimed using a process called lyophilization, also known as freeze drying. This is accomplished by freezing a water-filled flask in a dry ice acetone bath at -78 °C and then applying high vacuum by attachment to a lyophilizer, where the water is recaptured in a cold finger.
Many mothballs contain a compound known as naphthalene, which is a simple polyaromatic hydrocarbon, consisting of two fused benzene rings.
Naphthalene sublimes at atmospheric pressure and 80 °C and the gaseous form of this compound is toxic to moths.
You’ve just watched JoVE’s introduction to Sublimation of Ferrocene. You should now understand the principles of sublimation, how to perform an experiment, and several of its applications. Thanks for watching!
