7.8:
The Uncertainty Principle
An electron is a subatomic particle with mass, m. But it also behaves as a wave, with velocity v, as demonstrated by the de Broglie relation. So, an electron has both wave- and particle-like characteristics.
Unfortunately, it is not possible to witness the electron being both a particle, with a defined location, and a wave, with a known velocity or momentum, at the same time. What happens when an experiment is set up to observe the dual nature of an electron?
First, reconsider the double-slit experiment where there are two closely-spaced apertures. When a beam of electrons passes through the slits, an interference pattern is produced. This is a unique property of waves.
When electrons pass through one by one, the same pattern is observed. Since an electron is a particle, it should be possible to monitor which slit or slits it travels through.
To study this, a laser beam is arranged directly behind the slits. When an electron travels through a slit, it produces a small flash — indicating the slit it just passed through.
During the experiment, flashes are observed at only one of the slits at a time, but never both slits simultaneously. Moreover, the interference pattern is no longer observed; instead, two bright lines are seen. In trying to observe the particle-nature of the electron, its wave nature is lost.
In other words, the electron is observed as either a particle or a wave, but never both at the same time. The particle-nature and wave-nature of an electron, and by extension its position and momentum, are therefore complementary properties. This means that it is also impossible to simultaneously observe the accurate position and velocity of an electron.
Werner Heisenberg related that the uncertainty in these properties, represented by Δx and mΔv, must be greater than or equal to a finite quantity — Planck’s constant over 4π. This is known as Heisenberg’s Uncertainty Principle.
The more accurately the position of the electron is known, and the smaller the Δx, the less certain the velocity of the electron is known and larger the Δv, and vice versa.
Now, consider a golf ball resting on a tee. According to classical physics, the path or trajectory of the golf ball can be predicted by knowing its initial position, the force with which it is hit, and the effect of other factors, such as gravity, wind, and air resistance.
With these data, the golf ball’s position and velocity can be determined at any moment.
However, if only the golf ball’s initial location is known, it is not possible to deduce its final position.
Similarly, for an electron, because its position and velocity can not be known at the same time, its trajectory can also not be predicted. This is known as the indeterminacy behavior of an electron. Its present location can not determine its future position.
Because of this, instead of describing a precise position for an electron, the likelihood, or probability, of finding it within a certain region of the atom is used. This is known as a probability density, where each dot represents the potential location of an electron within an atom.
The density of dots is proportional to the probability of finding an electron. So, an electron is more likely to be found closer to the atom’s nucleus than very far away. Thus, a more accurate representation of the atom is depicted by the nucleus surrounded by its electron probability density, which is also known as the electron cloud model.
7.8:
The Uncertainty Principle
Werner Heisenberg considered the limits of how accurately one can measure properties of an electron or other microscopic particles. He determined that there is a fundamental limit to how accurately one can measure both a particle’s position and its momentum simultaneously. The more accurate the measurement of the momentum of a particle is known, the less accurate the position at that time is known and vice versa. This is what is now called the Heisenberg uncertainty principle. He mathematically related the uncertainty in the position and the uncertainty in momentum to the quantity involving Planck’s constant.
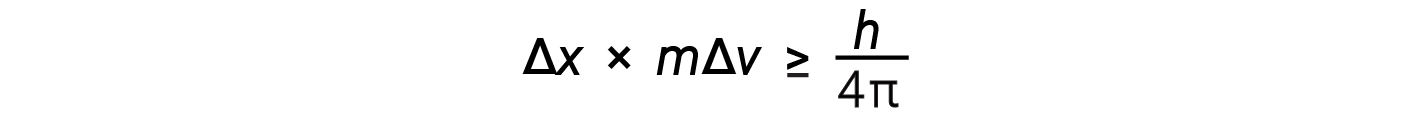
This equation calculates the limit to how precisely one can know both the simultaneous position of an object and its momentum.
Thus, the more accurate is the position of the electron, the less accurate is its velocity and vice versa. For example, one can predict where a baseball would land in the outfield by noting its initial position and velocity and by considering the effect of gravity and wind, etc. The trajectory of the baseball can be estimated.
For an electron, however, the position and velocity cannot be determined simultaneously. Therefore, a trajectory for the electron of an atom cannot be determined. This behavior is indeterminate. Instead of the precise location of an electron, one can talk in terms of the probability of finding an electron in a certain region of the atom, which is a probability density. It can be indicated as psi square (ψ2). The higher the probability of finding an electron in a particular region, the larger the value of psi square. Based on this, atoms are described as consisting of a nucleus surrounded by an electron cloud.
Heisenberg’s principle imposes ultimate limits on what is knowable in science. The uncertainty principle can be shown to be a consequence of wave–particle duality, which lies at the heart of what distinguishes modern quantum theory from classical mechanics.
This text is adapted from Openstax, Chemistry 2e, Section 6.3: Development of Quantum Theory.
