17.5:
反応における標準エントロピーの変化
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Core
Chemistry
Standard Entropy Change for a Reaction
Enthalpy changes associated with a chemical reaction can be measured with a calorimeter, but the entropy change associated with a reaction cannot be directly measured. Entropy is a state function, which means that the change in entropy depends solely on the initial and final states of a system. So, like enthalpy changes, entropy changes can be from calculated reference tables of standard molar entropies. For a reaction occurring under standard conditions, the associated entropy change is determined by the difference between the sum of the standard molar entropies of the products multiplied by their stoichiometric coefficients and the sum of the standard molar entropies of the reactants multiplied by their stoichiometric coefficients. Consider the combustion of ethylene under standard conditions, where 1 mole of ethylene gas reacts with 3 moles of oxygen gas to produce 2 moles of carbon dioxide gas and 2 moles of water. The standard entropy change for the reaction equals the sum of 2 times the standard entropy of carbon dioxide gas and 2 times the standard entropy of water, minus the sum of the standard entropy of ethylene gas and 3 times the standard entropy of oxygen. Note that, unlike standard enthalpies of formation of elements, which are zero, standard molar entropies of all substances are greater than zero at 298 K. Substituting the values for molar entropies of reactants and products from the reference table yields [(2 × 213.8) + (2 × 70.0)] − [(219.5 + 3) × (205.3)]. The net entropy of the products equals 567.6 J/K, and the net entropy of the reactants is 835.4 J/K. The difference between the products and the reactants equals negative 268 J/K for the standard entropy change of the combustion of ethylene. The negative value indicates there is a decrease in entropy. Even without calculating the exact entropy change, the decrease in entropy can be predicted by examining the reaction. Recall that gases are more disordered than liquids. There are more moles of gas in the reactants, 4 moles of gas (with 1 mole of ethylene and 3 moles of oxygen) compared to the products (only 2 moles of carbon dioxide gas), while the other product is a liquid. Thus, in this reaction, the reactants are more disordered than the products. Therefore, entropy decreases as the reaction proceeds.
17.5:
反応における標準エントロピーの変化
エントロピーは状態関数なので、化学反応の標準エントロピー変化( ΔS°rxn )は、生成物と反応物の標準エントロピーの差から計算できます。
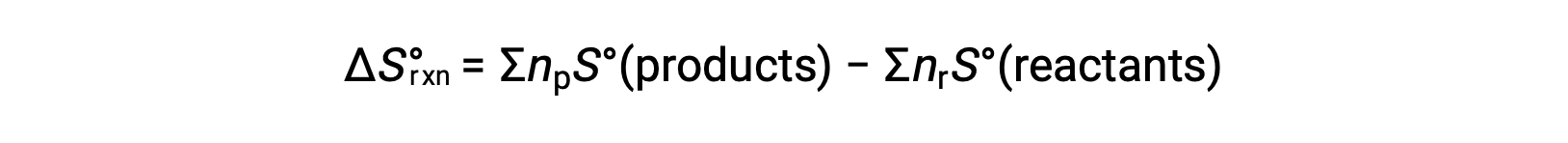
ここで、npとnrは、それぞれ生成物と反応物の平衡式における化学量論的な係数を表します。
例えば、室温での次のような反応における ΔS°rxn
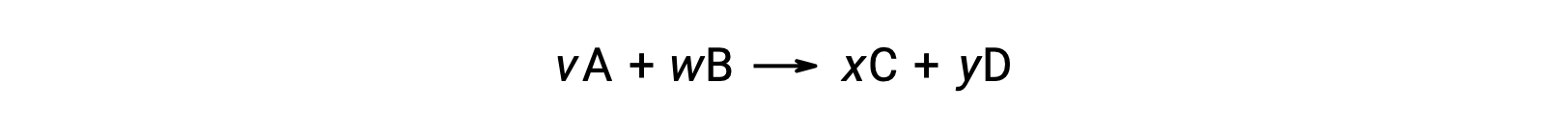
は、次のように計算できます。
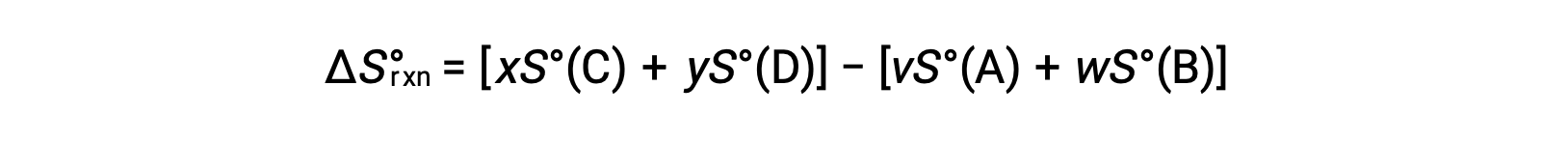
標準的なエントロピーの一部を表にまとめました。
| 物質 | S° (J/mol·K) |
| C (s, graphite) | 5.740 |
| C (s, diamond) | 2.38 |
| CO (g) | 197.7 |
| CO2 (g) | 213.8 |
| CH4 (g) | 186.3 |
| C2H4 (g) | 219.5 |
| C2H6 (g) | 229.5 |
| CH3OH (l) | 126.8 |
| C2H5OH (l) | 160.7 |
| H2 (g) | 130.57 |
| H (g) | 114.6 |
| H2O (g) | 188.71 |
| H2O (l) | 69.91 |
| HCI (g) | 186.8 |
| H2S (g) | 205.7 |
| O2 (g) | 205.03 |
ΔS°の決定
気体のH2O 1 molが液体のH2O 1 molに変化する水の凝縮を考えます。
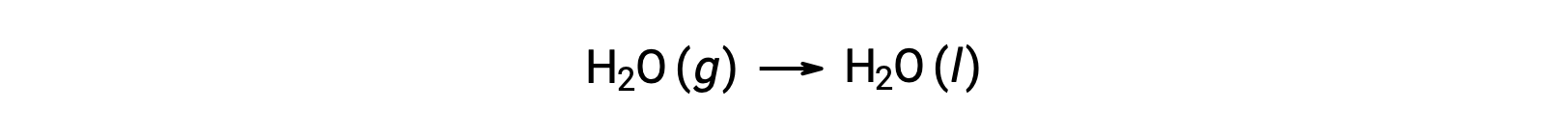
反応に対する標準エントロピー変化である ΔS°rxn を、標準モルエントロピーと化学量論的係数を用いて計算します。
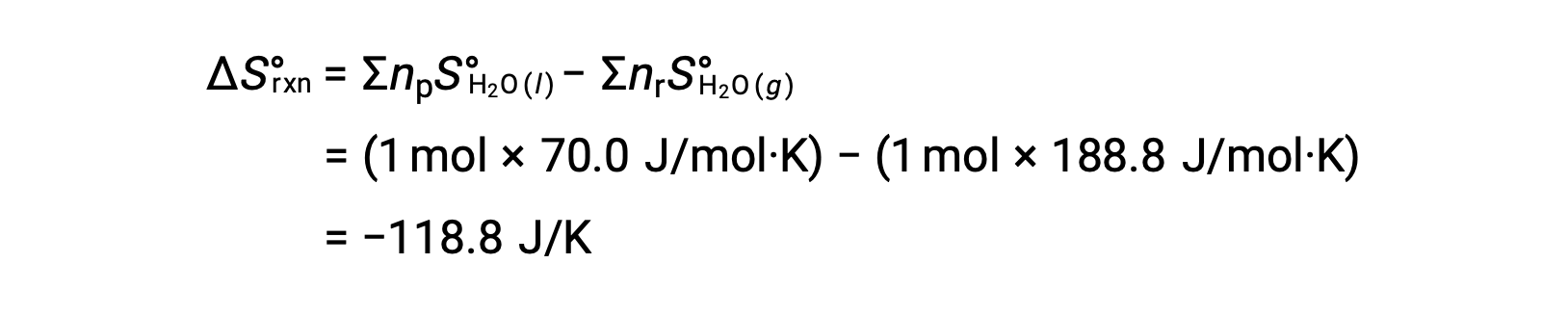
ΔS°rxnの値は、この相転移(凝縮)で予想されるように負の値を示しています。
2つ目の例として、メタノール(CH3OH)の燃焼を考えます。
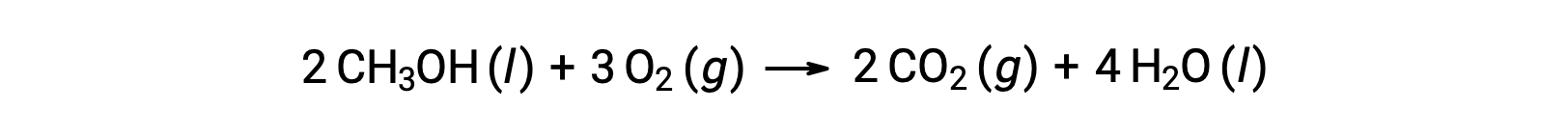
同様の手順で、反応の標準エントロピー変化を計算すると、次のようになります。
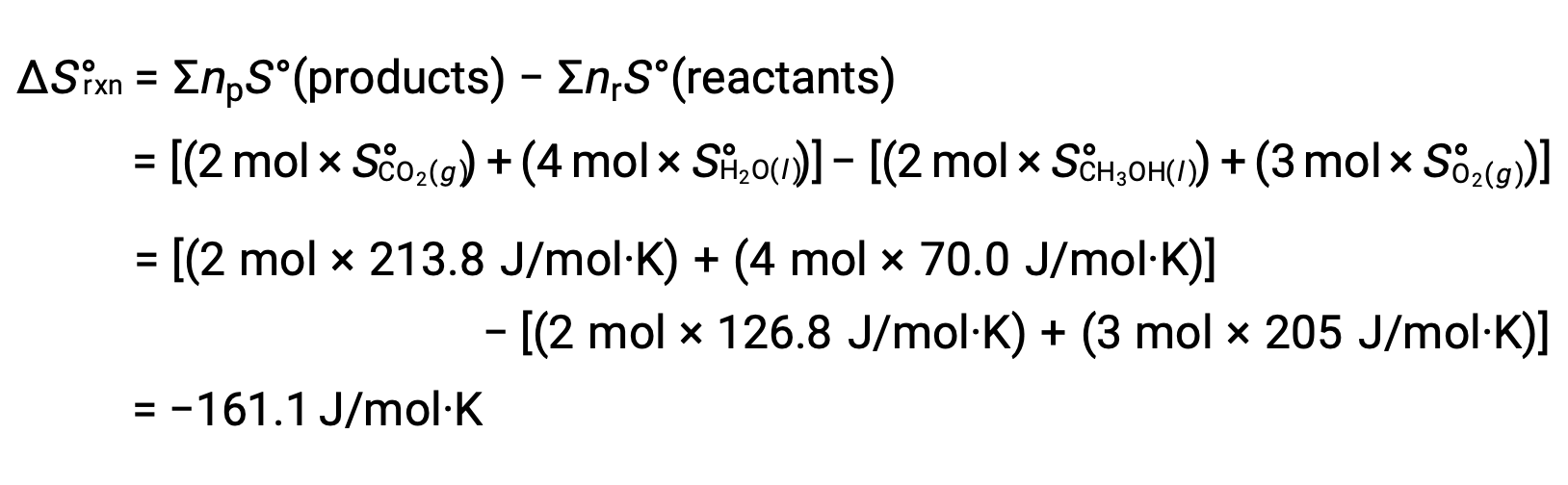
上記の文章は以下から引用しました。Openstax, Chemistry 2e, Chapter 16.2: The Second and Third Law of Thermodynamics.
