重结晶法净化化合物
English
Share
Overview
资料来源: 实验室的博士吉米 · 佛朗哥-梅里马克大学
再结晶是一种用于净化固体化合物技术。1固体倾向于更溶于热的液体比冷液体中。在再结晶,不纯的固体化合物溶解在热的液体直到饱和溶液,然后允许的液体冷却。2该化合物应该然后形成相对纯净的晶体。理想情况下,存在任何杂质始终在解决方案中,将不会合并成生长晶体 (图 1)。晶体,可以用过滤法去除从解决方案。不是所有的这种化合物是可采 — — 部分将留在解决方案中,而且将会丢失。
再结晶是不普遍认为是一种分离技术;相反,它是极少量的杂质从一种化合物的纯化技术。然而,如果两种化合物的溶解性能足够不同,再结晶可用于将它们分开,即使他们目前在几乎相等的金额。时的大部分杂质已被删除的其他方法,如提取或柱层析,再结晶的效果最好。
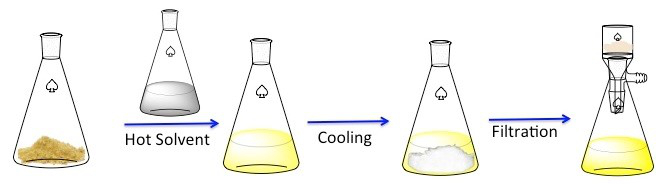
图 1。再结晶的一般方案。
Principles
成功的再结晶取决于溶剂的正确选择。冷的时候,必须溶于热溶剂,不溶于同一溶剂化合物。再结晶,否 3 %w / v 的分界线之间可溶性和不溶性: 如果 3g 的一种化合物溶解在 100 毫升的一种溶剂,它被认为是可溶性。在选择从再结晶溶剂,热溶解度和冷溶解度之间的差异越大,可恢复更多的产品。
冷却速率确定大小和晶体质量: 快速冷却有利于小水晶,和缓慢冷却有利于大和一般纯净的晶体的生长。再结晶速度通常最伟大的约 50 ° C 低于熔点的物质;晶体的最大形成发生在约 100 ° C 以下的熔点。
虽然有时交替使用的术语”结晶”和”结晶”,他们从技术上讲是指不同的过程。结晶是指形成新的、 不溶性的产品的一种化学反应;本产品然后沉淀反应解决方案作为包含很多被困的杂质无定形固体。再结晶并不涉及化学反应;粗品简单地溶解,然后改变条件允许,重新形成的晶体。再结晶产生更纯的最终产物。为此,通常采用结晶法生产固体产物的实验程序包括一个最后再结晶步骤给纯化合物。
Procedure
Results
An example of the results of recrystallization is shown in Figure 2. The yellow impurities present in the crude compound have been removed, and the pure product is left as an off-white solid. The purity of the recrystallized compound can now be verified by nuclear magnetic resonance (NMR) spectroscopy or, if it is a compound with a published melting point, by how similar its melting point is to the literature melting point. If necessary, multiple recrystallizations can be performed until the purity is acceptably high.

Figure 2. 2a) A crude compound (left), 2b) recrystallized product before filtration (middle), and 2c) the same compound after recrystallization (right).
Applications and Summary
Recrystallization is a method of purifying a compound by removing any impurities that might be mixed with it. It works best when the compound is very soluble in a hot solvent, but very insoluble in the cold version of the same solvent. The compound must be a solid at room temperature. Recrystallization is often used as a final clean-up step, after other methods (such as extraction or column chromatography) that are effective at removing larger amounts of impurities, but that do not raise the purity of the final compound to a sufficiently high level.
Recrystallization is the only technique that can produce absolutely pure, perfect single crystals of a compound. These crystals can be used for X-ray analysis, which is the ultimate authority in determining the structure and three-dimensional shape of a molecule. In these cases, the recrystallization is allowed to proceed very slowly, over the course of weeks to months, to allow the crystal lattice to form without the inclusion of any impurities. Special glassware is needed to allow the solvent to evaporate as slowly as possible during this time, or to allow the solvent to very slowly mix with another solvent in which the compound is insoluble (called antisolvent addition).
The pharmaceutical industry also makes heavy use of recrystallization, since it is a means of purification more easily scaled up than column chromatography.3 The importance of recrystallization in industrial applications has triggered educators to emphasize recrystallization in the laboratory curriculum.4 For example, the drug Stavudine, which is used to reduce the effects of HIV, is typically isolated by crystallization.5 Often, molecules have multiple different crystal structures available, so it is necessary for research to evaluate and understand which crystal form is isolated under what conditions, such as cooling rate, solvent composition, and so forth. These different crystal forms might have different biological properties or be absorbed into the body at different rates.
A more common use of recrystallization is in making rock candy. Rock candy is made by dissolving sugar in hot water to the point of saturation. Wooden sticks are placed into the solution and the solution is allowed to cool and evaporate slowly. After several days, large crystals of sugar have grown all over the wooden sticks.
Disclosures
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
References
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
Transcript
Recrystallization is a purification technique for solid compounds.
To perform recrystallization, an impure solid compound is mixed with hot solvent to form a saturated solution. As this solution cools, the solubility of the compound decreases, and pure crystals grow from solution.
Recrystallization is often used as a final step after other separation methods such as extraction, or column chromatography. Recrystallization may also be used to separate two compounds with very different solubility properties. This video will illustrate solvent selection for recrystallization, purification of an organic compound from solution, and will introduce a few applications in chemistry.
Crystallization begins with nucleation. Solute molecules come together to form a stable small crystal, which is followed by crystal growth. Nucleation occurs faster on nucleation sites such as seed crystals, scratches, or solid impurities than spontaneously in solution. Agitation may also encourage rapid nucleation. However, rapid growth can lead to incorporation of impurities if not grown in optimal conditions.
The solubility of a compound tends to increase with temperature, and is highly dependent on the choice of solvent. The greater the difference in solubility at high and low temperature, the more likely it is for the solute to come out of the solution as it cools, and form crystals.
The solvent chosen should have a boiling point of at least 40 °C so there is a significant temperature difference between boiling and room temperature. The solvent’s boiling point must also be below the melting point of the solute to enable crystallization. Rapid cooling of the solution induces the formation of many nucleation sites, thus favors the growth of many small crystals. However, slow cooling induces the formation of fewer nucleation sites, and favors larger and purer crystals. Thus, slow cooling is preferred.
Additionally, a solvent can be selected to minimize impurities. If a solution impurity is more soluble than the solute itself, it can be washed off of the fully formed crystals with cold solvent. However, if an impurity is less soluble, it will crystalize first, and can then be filtered out of the heated solution, prior to recrystallization of the solute.
If no single solvent has the necessary properties, a mixture of solvents can be used. For a solvent pair, the first solvent should readily dissolve the solid. The second solvent must have a lower solubility for the solute and be miscible with the first solvent. Common solvent pairs include ethyl acetate and hexane, toluene and hexane, methanol and dichloromethane, and water and ethanol.
Now that you understand the principles of recrystallization, let’s go through a procedure for purification of an organic compound by recrystallization.
To begin this procedure, place 50 mg of the sample in a glass test tube.
Add 0.5 mL of room temperature solvent. If the compound dissolves completely, the solubility in the cold solvent is too high to be used for recrystallization. Otherwise, heat the mixture in the test tube to boiling.
If the compound does not dissolve completely in the boiling solvent, heat another portion of solvent to boiling. Add the boiling solvent dropwise to the test tube until the solid dissolves completely or until the test tube contains 3 mL of solvent. If the solid still does not dissolve, then its solubility in this solvent is too low.
Confirm that impurities are either insoluble in the hot solvent so they can be filtered out after dissolution or soluble in the cold solvent so they remain in solution after recrystallization is complete. If a solvent meets all criteria, it is suitable for recrystallization.
To start recrystallization, heat the solvent to boiling on a hot plate in an Erlenmeyer flask with a stir bar. Place the compound to be recrystallized in another Erlenmeyer flask at room temperature.
Next, add a small portion of hot solvent to the compound. Swirl the mixture in the flask and then place it on the hot plate as well. Repeat this process until the sample has completely dissolved or until addition of solvent causes no further dissolution.
Add a 10% excess of hot solvent to the solution to account for evaporation. Place filter paper in a Büchner funnel setup. Filter the solution to remove insoluble impurities. If crystals form during filtration, dissolve them with drops of hot solvent.
Cool the solution on the benchtop. Cover the flask to prevent solvent loss to evaporation and to keep particulates out of the solution.
Leave the flask undisturbed until it has cooled to room temperature. Agitation during cooling may cause rapid crystallization, yielding less pure crystals. If no crystal formation is evident upon cooling, induce crystallization by gently scratching the inside walls of the flask with a glass rod or adding a small seed crystal of the compound being recrystallized.
If crystal formation cannot be induced, reheat the solution to boil off some of the solvent, and then cool the solvent to room temperature once more.
Once crystals have formed, prepare an ice bath. Keeping the solution covered, cool the solution in the ice bath until crystallization appears to be complete.
Clamp a filtration flask to a ring stand and connect the flask to a vacuum line. Set a Büchner funnel and adapter in the mouth of the flask.
Pour the mixture of solution and crystals into the funnel and begin vacuum filtration. Rinse any crystals remaining in the flask into the funnel with cold solvent. Wash the crystals on the funnel with cold solvent to remove soluble impurities.
Continue drawing air through the funnel to dry the crystals and then turn off the vacuum pump. If necessary, the crystals may be allowed to stand at room temperature to air dry or placed in a desiccator before storing the crystallized solid.
The yellow impurities present in the crude compound have been removed, yielding an off-white solid. Based on the identity of the compound and the impurities, the purity of the crystals can be verified by NMR spectroscopy, melting point measurements, or visual inspection.
Purification by recrystallization is an important tool for chemical synthesis and analysis.
X-ray crystallography is a powerful characterization technique that identifies the three-dimensional atomic structure of a molecule. This requires a pure single crystal, which is obtained by recrystallization. Some classes of molecules such as proteins are difficult to crystallize, but their structures are extremely important for understanding their chemical functions. With careful selection of recrystallization conditions, even these classes of molecules can be analyzed by X-ray crystallography. To learn more about this process, see this collection’s video on growing crystals for crystallography.
Impure reactants can cause unwanted side reactions. Purifying reactants by recrystallization improves product purity and yield. Once a solid product has been isolated and washed, reaction yield can also be increased by removing volatiles from the filtrate and recrystallizing the product from the resulting solid. Antifreeze proteins, or AFPs, are expressed in many organisms that live in icy environments. AFPs hinder internal ice growth by binding to ice planes, inhibiting recrystallization into larger ice crystals. Different AFPs bind to different types of ice crystal planes. Investigating AFP binding mechanisms involves adsorbing them onto single ice crystals. Proper growth of a single ice crystal is essential for clear and informative results. These proteins have applications from the engineering of cold-resistant crops to cryosurgery.
You’ve just watched JoVE’s introduction to purifying compounds by recrystallization. You should now be familiar with the principles of the technique, a purification procedure, and some applications of recrystallization in chemistry.
Thanks for watching!
