16.11:
Factors Affecting Solubility
16.11:
Factors Affecting Solubility
Compared with pure water, the solubility of an ionic compound is less in aqueous solutions containing a common ion (one also produced by dissolution of the ionic compound). This is an example of a phenomenon known as the common ion effect, which is a consequence of the law of mass action that may be explained using Le Chȃtelier’s principle. Consider the dissolution of silver iodide:
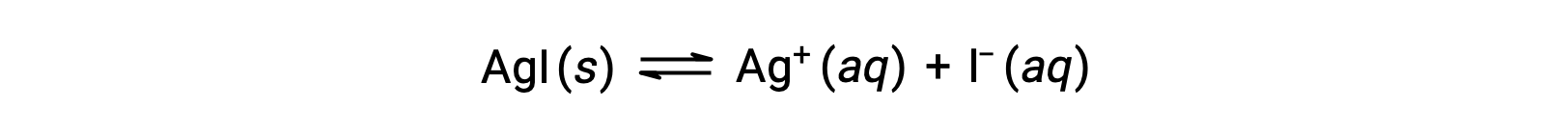
This solubility equilibrium may be shifted left by the addition of either silver or iodide ions, resulting in the precipitation of AgI and lowered concentrations of dissolved Ag+ and I–. In solutions that already contain either of these ions, less AgI may be dissolved than in solutions without these ions.
This effect may also be explained in terms of mass action as represented in the solubility product expression:
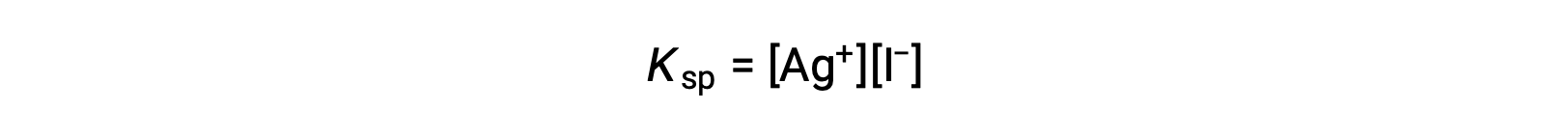
The mathematical product of silver and iodide ion molarities is constant in an equilibrium mixture regardless of the source of the ions, and so an increase in one ion’s concentration must be balanced by a proportional decrease in the other.
The Role of Precipitation in Wastewater Treatment
Solubility equilibria are useful tools in the treatment of wastewater carried out in facilities that may treat the municipal water in a city or town. Specifically, selective precipitation is used to remove contaminants from wastewater before it is released back into natural bodies of water. For example, phosphate ions (PO43−) are often present in the water discharged from manufacturing facilities. An abundance of phosphate causes excess algae to grow, which impacts the amount of oxygen available for marine life as well as making the water unsuitable for human consumption.
One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, Ca(OH)2. As the water is made more basic, the calcium ions react with phosphate ions to produce hydroxylapatite, Ca5(PO4)3·OH, which then precipitates out of the solution:
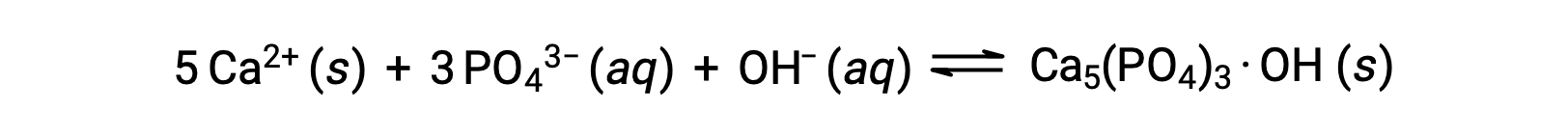
Because the amount of calcium ion added does not result in exceeding the solubility products for other calcium salts, the anions of those salts remain behind in the wastewater. The precipitate is then removed by filtration, and the water is brought back to a neutral pH by the addition of CO2 in a recarbonation process. Other chemicals can also be used for the removal of phosphates by precipitation, including iron(III) chloride and aluminum sulfate.
This text is adapted from Openstax, Chemistry 2e, Section 15.1: Precipitation and Dissolution.
Suggested Reading
- Koubek, E. "Demonstration of the Common Ion Effect." Journal of chemical education 70, no. 2 (1993): 155.
- Amaral, L. F., I. R. Oliveira, R. Salomão, E. Frollini, and V. C. Pandolfelli. "Temperature and common-ion effect on magnesium oxide (MgO) hydration." Ceramics International 36, no. 3 (2010): 1047-1054.
- Cassens, Jan, Anke Prudic, Feelly Ruether, and Gabriele Sadowski. "Solubility of pharmaceuticals and their salts as a function of pH." Industrial & Engineering Chemistry Research 52, no. 7 (2013): 2721-2731.
