18.2:
Electromotive Force
18.2:
Electromotive Force
Electricity is generated by either electrons or ions flowing through a solution or a conducting medium. This flow of electrons or specifically electrical charge is defined as an electric current. When electrons move through a wire, they generate an electric current. It can be recalled that in a redox reaction, electrons are lost and gained. In the spontaneous redox reaction of zinc with copper, when zinc is immersed in a copper ion solution, a transfer of electrons from one substance to another occurs.
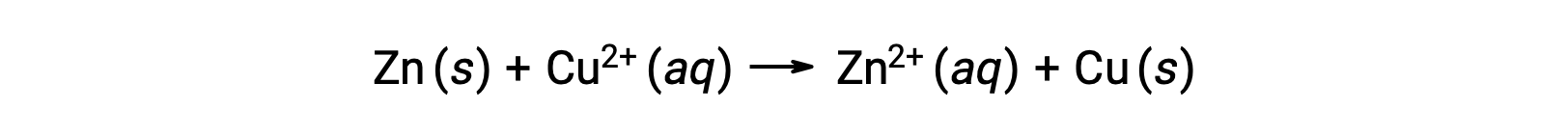
Zinc, having a greater tendency to lose electrons, is oxidized to zinc ions, while copper ions are reduced to solid copper. However, this reaction does not generate electricity.
Electrical Current and How Electrons Flow
Electron transfer occurs directly from a reducing agent to an oxidizing agent in a solution. Even if the components of half-reactions are physically isolated in separate vessels and connected via an external conductor such as a wire, the tendency to lose and gain electrons by the reactants still persists. However, now, electrons are forced to flow through the wire connecting the two half-reactions. This electron flow through the wire constitutes an electric current and can power electronic appliances, such as a light-bulb. Electric current is measured in amperes. One ampere is equal to the flow of one coulomb of electrical charge per second and is equal to 6.24 × 10−18 electrons per second.
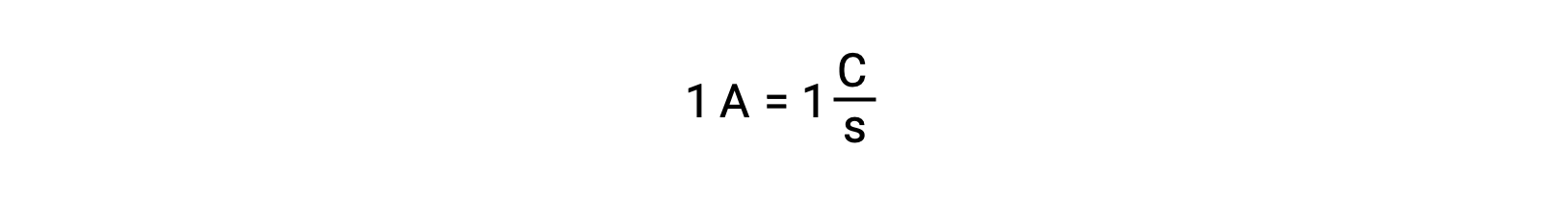
Since an electron has a charge of 1.602 × 10−19 C, 1 ampere correlates to the flow of 6.242 × 1018 electrons per second.
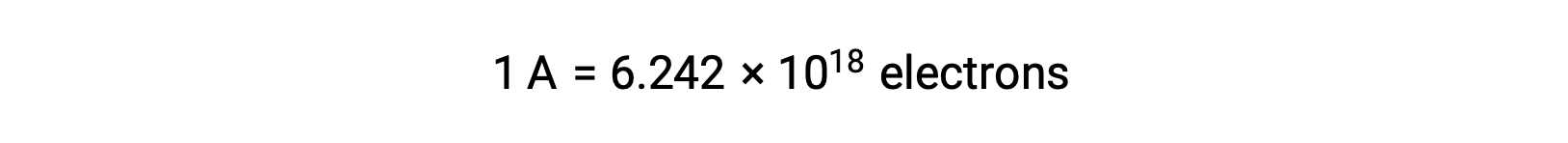
Driving Force for Electrical Current, Potential Difference, and Emf
The flow of electrical current is similar to water flowing down a waterfall. The water is driven by the difference in gravitational potential energy, while the flow of electrons is driven by the difference of the electrical potential energy between the reactants. This difference in electrical potential energy is described either by the terms potential difference, electromotive force (emf), or cell potential. The emf is a measure of the driving force between two reactants and the tendency for electron transfer.
Some redox reactions are spontaneous, while others are not. For example, a copper wire undergoes spontaneous oxidation by silver(I) ions, but fails to yield any reaction when immersed in a solution of lead(II) ions. This is due to the difference in the redox activity of the two species, Ag+ (aq) and Pb2+ (aq), towards copper: the silver ion spontaneously oxidizes copper, but the lead ion does not. This difference in redox reactivity in electrochemistry can be quantified using the term ‘cell potential’; also commonly known as ‘voltage’.
The cell potential of two isolated reactants is measured with a voltmeter, which is read in cell voltage. One volt correlates to one joule of potential energy per one coulomb of electrical charge.
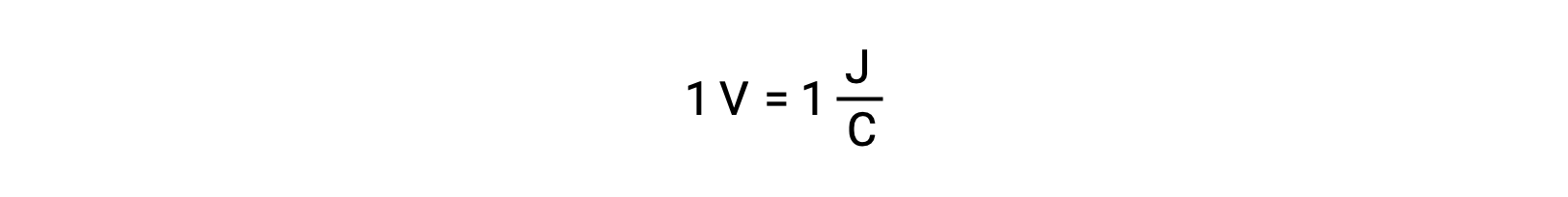
A high cell potential indicates a large driving force and greater ease of electron transfer. Lastly, the electromotive force, or cell potential, depends on the reactants’ nature, the reaction temperature, and the concentration of ions present in the reaction.
This text is adapted from OpenStax, Chemistry 2e, Section 17.3: Electrode and Cell Potentials.
