转换的脂肪酸甲酯的皂化为 Uk'37 Paleothermometry
English
Share
Overview
资料来源: 实验室的杰夫卡普-马萨诸塞大学阿默斯特
有机溶剂萃取,总脂提取物 (TLE) 产品经常是几百,如果不是,数千种不同化合物的复杂混合物。研究者往往只是一小撮化合物有兴趣或如果都感兴趣,可能需要删除不需要的成分,都是”以”或共同洗脱。例如,单个化合物样品中的浓度往往决意对耦合到火焰电离检测器 (GC) 气相色谱仪因为 FID 响应 (在 pA) 和化合物 (如、 吴/μ) 样品中的量之间的关系是线性和敏感。该仪器的 GC 部分分离不同化合物在基于其沸点、 化学结构和亲和力与固相,可以随应用程序的示例。其结果是色谱图 (图茜 1),显示不同化学成分的分离时间,以及它们的相对浓度 (作为曲线下面积计算)。然而,有时还不止一种化合物洗脱了 GC (无花果茜 1) 的一次。在这种情况下,样品净化是需要之前化合物可以自信地量化。
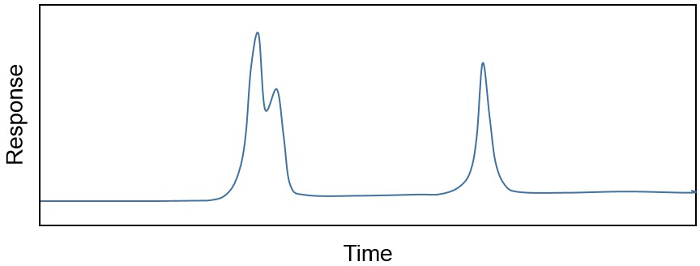
图 1.色谱图显示不同的化学成分随时间和它们的相对浓度 (曲线下的面积) 的分离。显示共同洗脱和失散的山峰。
Principles
Procedure
Results
This purification produces a TLE free of esters that may be co-eluting with the alkenones. However, the purification produced carboxylic acids, which cannot be injected onto instruments commonly used to analyze samples for alkenone concentrations because of their low volatility. For example, the boiling point of hexane, a 6-carbon hydrocarbon, is 68 ˚C, but the boiling point of its acid (hexanoic acid) is 205 ˚C. Most GC amenable biomarkers have from 20 to 35 carbon atoms (boiling point generally increases with an increasing number of atoms), and most GC temperature programs stop around 300 ˚C. Carboxylic acids injected into a GC quickly accumulate and ruin inlets, inlet liners, and the front end of columns. To remove these acids, the sample first needs to undergo another purification technique separation via column chromatography.
Applications and Summary
As mentioned previously, saponification is commonly used in organic geochemistry labs to remove fatty acid methyl esters (FAMEs) of alkenones, called alkenoates, which co-elute with alkenones on gas chromatographs (Figure 1). Saponification is also used to "free" fatty acids "bound" to sediment or macromolecules.The degradation and preservation of organic matter and biomarkers in sediments involves the removal of functional groups (N, O, and S) and the eventual polymerization of individual biomarkers into macromolecules and/or the adsorption of biomarkers and macromolecules onto mineral surfaces. Not all biomarkers in a setting become bound, and the ratio of bound-to-free biomarkers may change by setting and sediment age for reasons still not fully explained. Saponification, sometimes at temperatures higher than those discussed here (>200 ˚C), is used to free these bound biomarkers in the effort to describe them, their source, and the mechanisms responsible for their bound nature.
Transcript
Saponification is a technique commonly used to remove fatty acid methyl esters from a complex organic mixture.
Complex organic samples are often analyzed by gas chromatography, which is used to determine the relative concentrations of individual components.
However, compounds that are similar in size and structure cannot be distinguished by the instrument, skewing the results. Therefore, unwanted compounds that produce overlapping signals must be removed in order to obtain accurate results.
This video covers the use of saponification to purify alkenones for paleoclimatology. It is the first in a series detailing the purification of complex biomarker samples. It will cover the procedure, as well as some other uses of the technique.
Polyunsaturated long chain alkyl ketones, called alkenones, have been shown to provide useful information about past sea surface temperature.
However, the organisms that produce alkenones often create fatty acid methyl esters that are similar in size and chemical structure, called alkenoates. Because of these similarities, alkenoates must be removed before an accurate analysis can be obtained.
Saponification is a common technique used to prevent co-elution of these molecules. Saponification utilizes water to split the molecular bond of an ester. A base attaches to the carbon at the heart of the alkenoate. This addition reaction creates an unstable intermediate, and the alkoxide is expelled.
The hydrogen from the newly formed acid moves to the expelled alkoxide, and the resulting carboxylate anion forms an ionic bond with the cation from the base. The result is an alcohol and a fatty acid salt. Adding a strong acid will re-generate the carboxylic acid. At this point, the offending alkenoate has been converted to a form that no longer co-elutes with the alkenone of interest.
Now that you understand how saponification can be used to purify an organic mixture, you are ready to begin the procedure.
First, acquire a dried total lipid extract – or TLE – that was obtained using a solvent extraction method. Next, prepare the saponification solutions as outlined in the text protocol. Ensure that all components are pure and free of hydrocarbons. Once the solutions are prepared, add the dried TLE to a 60-mL borosilicate glass vial. Add 2 mL of 2 normal potassium hydroxide, and seal the vial. Next, heat the vial to 60 °C for 2.5 h to cleave the ester bond. When this is complete, allow the sample to cool to room temperature. Once the sample has cooled, add 2 mL of 5% sodium chloride solution to the vial. Cap the vial, and shake briefly. Add 6 N hydrochloric acid dropwise until a pH of 2 is reached – using pH paper to test. The addition of this acid will protonate the carboxylate anion to form the final product – the stable carboxylic acid.
Now that the acidified solution is ester-free, add 5 mL of hexane. Cap and shake vigorously for 5 seconds to extract the organic compounds from the water. Allow the solution to rest until the organic and aqueous phases separate completely. Salts, ions, and unreacted hydrochloric acid will remain in the aqueous phase, while organic compounds will separate into the hexane. Once the phases have completely separated, remove approximately 75% of the hexane using a pipette, and dispense into another 40-mL vial. Repeat this extraction process two additional times, adding 5 mL of hexane each time. Once this is complete, discard the leftover aqueous solution into a proper waste container. Label the vial containing the freshly saponified organic phase. The carboxylic acids produced cannot be injected into the instruments commonly used for alkenone analysis without further purification. Carboxylic acids injected into a gas chromatograph quickly accumulate and ruin inlets, inlet liners, and the front end of the columns. To remove these acids, the sample first needs to undergo another purification technique: separation via column chromatography.
Saponification has several applications in the extraction and purification of organic molecules.
Saponification can be used not only to separate biomarkers, but also to extract individual components for use in commercial products. In this example, compounds from the tobacco tree – Nicotiana glauca – were isolated and analyzed to investigate their potential as feedstock for a wide variety of bio-based products, such as fuel, heat, and an array of chemical compounds.
The leaves were first homogenized, then centrifuged, to concentrate the molecules of interest. The concentrated plant material was then saponified. The extracted material was analyzed with liquid chromatography-mass spectrometry, to determine the concentration of tocopherol – a family of vitamin E compounds typically found in plants.
The extracted material can also be reconstituted in vitro to its original composition. In this example, saponification was used to extract carotenoids from spinach plants, to be later reconstituted in vitro. The spinach was first homogenized, then centrifuged, to harvest the pigment molecules. These molecules were then suspended in a solution of potassium hydroxide in a separatory funnel, initiating saponification. The saponified carotenoids separated into an ether layer, which was collected and dried. Using a series of solution buffers, the carotenoids – and other pigmentation molecules – were later reconstituted in vitro. This purification allowed for analysis of these pigments without the interference of similarly structured organic compounds.
Because of its ability to hydrolyze esters, saponification can be used to “free” compounds that are otherwise bound to macromolecules. In this example, an anhydrous saponification step is used to convert ethyl 4-fluorobenzoate to the carboxylic acid salt of potassium4-fluorobenzoate. This deprotection through saponification allows for the production of crude SFB – a reaction that wouldn’t be possible had the molecule remained “stuck”.
You’ve just watched JoVE’s introduction to the purification of Uk’37 samples through saponification. You should now understand how saponification works, and how to use it to purify alkenones in a total lipid extract. Further purification processes will be demonstrated in subsequent videos.
Thanks for watching!
