Uk'37 고수온계의 비누화에 의한 지방산 메틸 에스테르의 전환
English
Share
Overview
출처: 제프 살라컵 연구소 – 매사추세츠 대학교 애머스트
유기 용매 추출의 제품, 총 지질 추출물 (TLE), 종종 수백의 복잡 한 혼합물, 하지 않을 경우 수천, 다른 화합물의. 연구원은 종종 화합물의 소수에 만 관심이 또는, 많은에 관심이 있는 경우, 원치 않는 성분을 제거 해야 할 수 있습니다”방법으로” 또는 동례. 예를 들어, 샘플내의 개별 화합물의 농도는 종종 불꽃 이온화 검출기(GC-FID)와 결합된 가스 크로마토그래프상에 결정되는데, 이는 FID 반응(pA)과 샘플내 화합물의양(예를 들어,ng/μL)의 관계가 선형및 민감하기 때문이다. 계측기의 GC 부분은 비등점, 화학 구조 및 응용 프로그램에 따라 변경할 수 있는 고체 상과 친화성에 기초한 샘플내의 상이 다른 화합물을 분리합니다. 그 결과 크로마토그램(도1)은 상이한 화학 성분의 분리뿐만 아니라 상대적 농도(곡선 아래 영역으로 계산됨)를 나타낸다. 그러나, 때로는 한 번에 하나의 화합물 엘루트(도1). 이 경우 화합물을 자신있게 정량화하기 전에 시료 정화가 필요합니다.
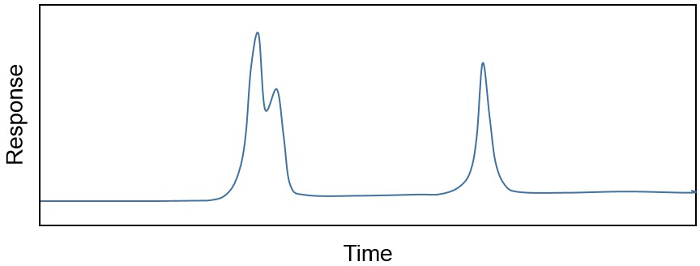
그림 1. 시간이 지남에 따라 다른 화학 성분의 분리와 상대 농도 (곡선 아래 영역)의 분리를 보여주는 크로마토그램. 동례 및 분리 된 피크가 표시됩니다.
Principles
Procedure
Results
This purification produces a TLE free of esters that may be co-eluting with the alkenones. However, the purification produced carboxylic acids, which cannot be injected onto instruments commonly used to analyze samples for alkenone concentrations because of their low volatility. For example, the boiling point of hexane, a 6-carbon hydrocarbon, is 68 ˚C, but the boiling point of its acid (hexanoic acid) is 205 ˚C. Most GC amenable biomarkers have from 20 to 35 carbon atoms (boiling point generally increases with an increasing number of atoms), and most GC temperature programs stop around 300 ˚C. Carboxylic acids injected into a GC quickly accumulate and ruin inlets, inlet liners, and the front end of columns. To remove these acids, the sample first needs to undergo another purification technique separation via column chromatography.
Applications and Summary
As mentioned previously, saponification is commonly used in organic geochemistry labs to remove fatty acid methyl esters (FAMEs) of alkenones, called alkenoates, which co-elute with alkenones on gas chromatographs (Figure 1). Saponification is also used to "free" fatty acids "bound" to sediment or macromolecules.The degradation and preservation of organic matter and biomarkers in sediments involves the removal of functional groups (N, O, and S) and the eventual polymerization of individual biomarkers into macromolecules and/or the adsorption of biomarkers and macromolecules onto mineral surfaces. Not all biomarkers in a setting become bound, and the ratio of bound-to-free biomarkers may change by setting and sediment age for reasons still not fully explained. Saponification, sometimes at temperatures higher than those discussed here (>200 ˚C), is used to free these bound biomarkers in the effort to describe them, their source, and the mechanisms responsible for their bound nature.
Transcript
Saponification is a technique commonly used to remove fatty acid methyl esters from a complex organic mixture.
Complex organic samples are often analyzed by gas chromatography, which is used to determine the relative concentrations of individual components.
However, compounds that are similar in size and structure cannot be distinguished by the instrument, skewing the results. Therefore, unwanted compounds that produce overlapping signals must be removed in order to obtain accurate results.
This video covers the use of saponification to purify alkenones for paleoclimatology. It is the first in a series detailing the purification of complex biomarker samples. It will cover the procedure, as well as some other uses of the technique.
Polyunsaturated long chain alkyl ketones, called alkenones, have been shown to provide useful information about past sea surface temperature.
However, the organisms that produce alkenones often create fatty acid methyl esters that are similar in size and chemical structure, called alkenoates. Because of these similarities, alkenoates must be removed before an accurate analysis can be obtained.
Saponification is a common technique used to prevent co-elution of these molecules. Saponification utilizes water to split the molecular bond of an ester. A base attaches to the carbon at the heart of the alkenoate. This addition reaction creates an unstable intermediate, and the alkoxide is expelled.
The hydrogen from the newly formed acid moves to the expelled alkoxide, and the resulting carboxylate anion forms an ionic bond with the cation from the base. The result is an alcohol and a fatty acid salt. Adding a strong acid will re-generate the carboxylic acid. At this point, the offending alkenoate has been converted to a form that no longer co-elutes with the alkenone of interest.
Now that you understand how saponification can be used to purify an organic mixture, you are ready to begin the procedure.
First, acquire a dried total lipid extract – or TLE – that was obtained using a solvent extraction method. Next, prepare the saponification solutions as outlined in the text protocol. Ensure that all components are pure and free of hydrocarbons. Once the solutions are prepared, add the dried TLE to a 60-mL borosilicate glass vial. Add 2 mL of 2 normal potassium hydroxide, and seal the vial. Next, heat the vial to 60 °C for 2.5 h to cleave the ester bond. When this is complete, allow the sample to cool to room temperature. Once the sample has cooled, add 2 mL of 5% sodium chloride solution to the vial. Cap the vial, and shake briefly. Add 6 N hydrochloric acid dropwise until a pH of 2 is reached – using pH paper to test. The addition of this acid will protonate the carboxylate anion to form the final product – the stable carboxylic acid.
Now that the acidified solution is ester-free, add 5 mL of hexane. Cap and shake vigorously for 5 seconds to extract the organic compounds from the water. Allow the solution to rest until the organic and aqueous phases separate completely. Salts, ions, and unreacted hydrochloric acid will remain in the aqueous phase, while organic compounds will separate into the hexane. Once the phases have completely separated, remove approximately 75% of the hexane using a pipette, and dispense into another 40-mL vial. Repeat this extraction process two additional times, adding 5 mL of hexane each time. Once this is complete, discard the leftover aqueous solution into a proper waste container. Label the vial containing the freshly saponified organic phase. The carboxylic acids produced cannot be injected into the instruments commonly used for alkenone analysis without further purification. Carboxylic acids injected into a gas chromatograph quickly accumulate and ruin inlets, inlet liners, and the front end of the columns. To remove these acids, the sample first needs to undergo another purification technique: separation via column chromatography.
Saponification has several applications in the extraction and purification of organic molecules.
Saponification can be used not only to separate biomarkers, but also to extract individual components for use in commercial products. In this example, compounds from the tobacco tree – Nicotiana glauca – were isolated and analyzed to investigate their potential as feedstock for a wide variety of bio-based products, such as fuel, heat, and an array of chemical compounds.
The leaves were first homogenized, then centrifuged, to concentrate the molecules of interest. The concentrated plant material was then saponified. The extracted material was analyzed with liquid chromatography-mass spectrometry, to determine the concentration of tocopherol – a family of vitamin E compounds typically found in plants.
The extracted material can also be reconstituted in vitro to its original composition. In this example, saponification was used to extract carotenoids from spinach plants, to be later reconstituted in vitro. The spinach was first homogenized, then centrifuged, to harvest the pigment molecules. These molecules were then suspended in a solution of potassium hydroxide in a separatory funnel, initiating saponification. The saponified carotenoids separated into an ether layer, which was collected and dried. Using a series of solution buffers, the carotenoids – and other pigmentation molecules – were later reconstituted in vitro. This purification allowed for analysis of these pigments without the interference of similarly structured organic compounds.
Because of its ability to hydrolyze esters, saponification can be used to “free” compounds that are otherwise bound to macromolecules. In this example, an anhydrous saponification step is used to convert ethyl 4-fluorobenzoate to the carboxylic acid salt of potassium4-fluorobenzoate. This deprotection through saponification allows for the production of crude SFB – a reaction that wouldn’t be possible had the molecule remained “stuck”.
You’ve just watched JoVE’s introduction to the purification of Uk’37 samples through saponification. You should now understand how saponification works, and how to use it to purify alkenones in a total lipid extract. Further purification processes will be demonstrated in subsequent videos.
Thanks for watching!
