18.7:
Concentration Cells
The cell potential of a redox reaction depends significantly on the concentration of the reactants and products.
Consider a nickel-silver galvanic cell under standard conditions with a cell potential of 1.03 volts. A change in concentration, however, can either increase or decrease the cell potential.
If a concentration gradient influences a cell potential of two different half-cells, can it be used to construct an electrochemical cell with identical half-reactions?
Consider a galvanic cell with two identical silver electrodes, each put in a solution containing a different concentration of silver ions. This set-up is called a concentration cell.
Following Le Châtelier's principle, the concentration gradient drives the electron flow spontaneously from the half-cell with the lower ion concentration to the half-cell with the higher ion concentration. Thus, oxidation occurs in the more dilute cell, where the silver electrode is oxidized, forming silver ions, whereas in the more concentrated cell, silver ions are reduced to solid silver.
The cell potential of a concentration cell is therefore determined solely by the concentration difference of the chosen redox reagent and can be calculated using the Nernst equation.
When the ion concentrations in the two half-cells become equal, the concentration cell reaches an equilibrium, and its potential becomes zero. At this point, the cell is pronounced ‘dead’.
pH meters operate using the same principle as a concentration cell to determine the acidity or basicity of a solution.
The glass electrode of the pH meter is filled with a solution of a known concentration of hydrogen ions. When immersed into a solution of a different hydrogen ion concentration, a measurable potential difference forms across the two sides of the glass and is used to determine the pH of the sample.
If the outside hydrogen ion concentration is higher than the inside of the electrode, the measured potential difference is high. This means that the solution is acidic with pH values below seven.
Equal hydrogen ion concentration on both sides results in a zero potential difference. Therefore, the measured solution is neutral. A lower hydrogen ion concentration on the outside generates a low potential difference, meaning the solution is basic with a pH value above seven.
18.7:
Concentration Cells
A concentration cell is a type of a voltaic cell constructed by connecting two almost identical half-cells, both based on the same half-reaction and using the same electrode, differing only in the concentration of one redox species. A concentration cell's potential, therefore, is determined only by the concentration difference of the particular redox species.
Consider the following voltaic cell:
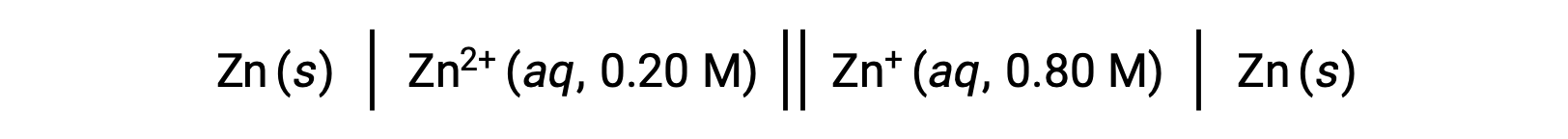
From the given information, the cell potential of this concentration cell can be calculated using the Nernst Equation:
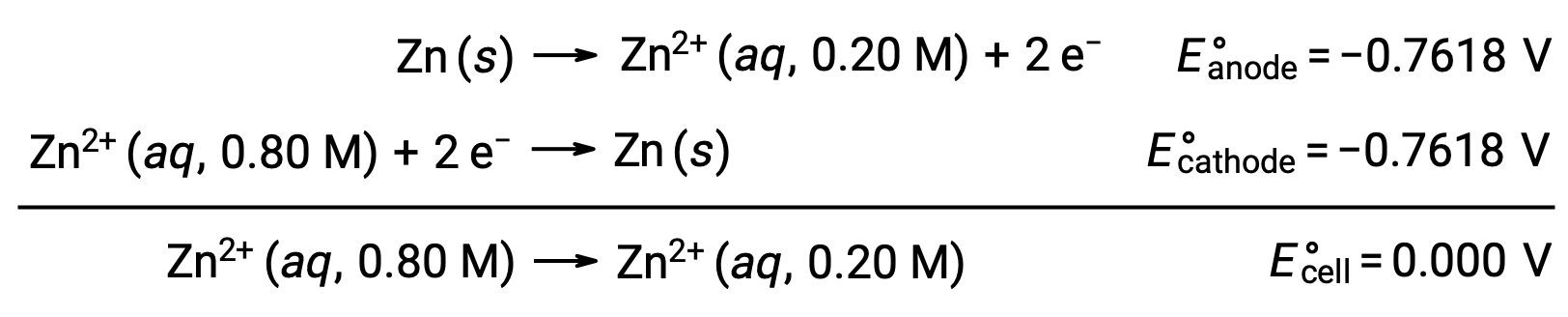
Substituting into the Nernst equation,
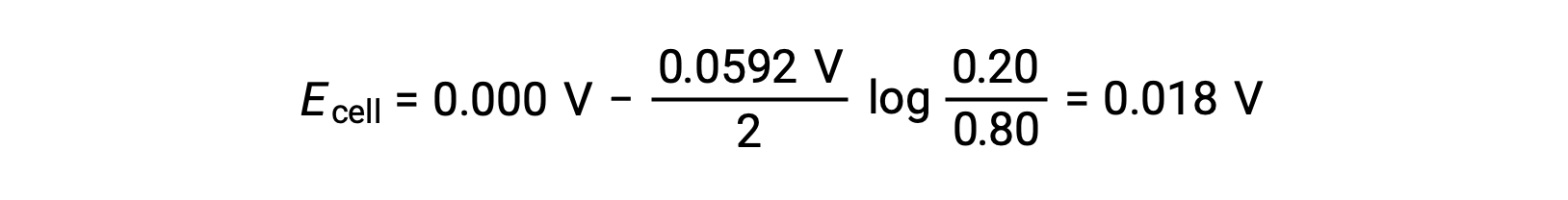
The positive value of the cell potential indicates that the overall cell reaction is spontaneous. This spontaneous reaction occurs when the zinc ion concentration in the cathode falls (by reduction to elemental zinc) while that in the anode rises (by oxidation of the zinc anode to zinc ions). A greater driving force for the reduction of zinc is present in the cathode, where the Zn2+ ion concentration is greater (Ecathode > Eanode).
pH meters in the lab, ion channels in the nerve cell membranes, and cardiac muscle cells in the human body work on the principle of concentration cells.
This text is adapted from Openstax,Chemistry 2e,Chapter 17.4: Potential, Free Energy, and Equilibrium.
