18.7:
濃淡電池
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Core
Chemistry
Concentration Cells
The cell potential of a redox reaction depends significantly on the concentration of the reactants and products. Consider a nickel-silver galvanic cell under standard conditions with a cell potential of 1.03 volts. A change in concentration, however, can either increase or decrease the cell potential. If a concentration gradient influences a cell potential of two different half-cells, can it be used to construct an electrochemical cell with identical half-reactions? Consider a galvanic cell with two identical silver electrodes, each put in a solution containing a different concentration of silver ions. This set-up is called a concentration cell. Following Le Châtelier's principle, the concentration gradient drives the electron flow spontaneously from the half-cell with the lower ion concentration to the half-cell with the higher ion concentration. Thus, oxidation occurs in the more dilute cell, where the silver electrode is oxidized, forming silver ions, whereas in the more concentrated cell, silver ions are reduced to solid silver. The cell potential of a concentration cell is therefore determined solely by the concentration difference of the chosen redox reagent and can be calculated using the Nernst equation. When the ion concentrations in the two half-cells become equal, the concentration cell reaches an equilibrium, and its potential becomes zero. At this point, the cell is pronounced ‘dead’. pH meters operate using the same principle as a concentration cell to determine the acidity or basicity of a solution. The glass electrode of the pH meter is filled with a solution of a known concentration of hydrogen ions. When immersed into a solution of a different hydrogen ion concentration, a measurable potential difference forms across the two sides of the glass and is used to determine the pH of the sample. If the outside hydrogen ion concentration is higher than the inside of the electrode, the measured potential difference is high. This means that the solution is acidic with pH values below seven. Equal hydrogen ion concentration on both sides results in a zero potential difference. Therefore, the measured solution is neutral. A lower hydrogen ion concentration on the outside generates a low potential difference, meaning the solution is basic with a pH value above seven.
18.7:
濃淡電池
濃淡電池とは、ほぼ同一の半反応を持つ2つの半電池を同じ電極で接続した電池の一種で、1つの酸化還元種の濃度が異なるだけです。したがって、濃淡電池の電位は、特定の酸化還元種の濃度差によってのみ決定されます。
次に示す電池を考える:
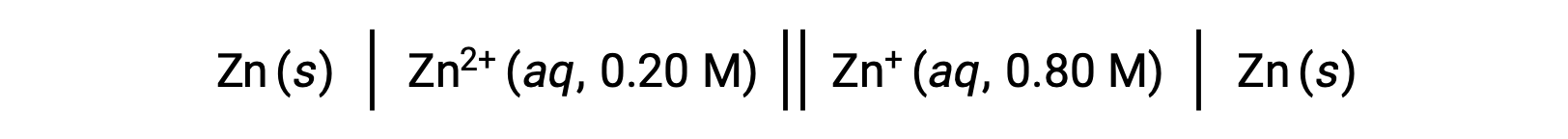
ネルンスト式を用いて、与えられた情報からこの濃淡電池の電極電位を計算することができます。
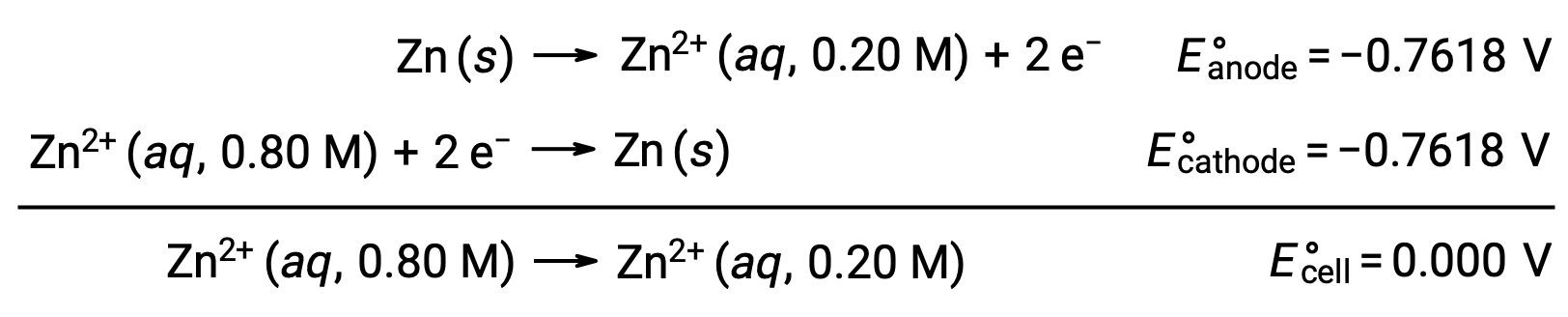
ネルンスト式に代入すると、次のようになります。
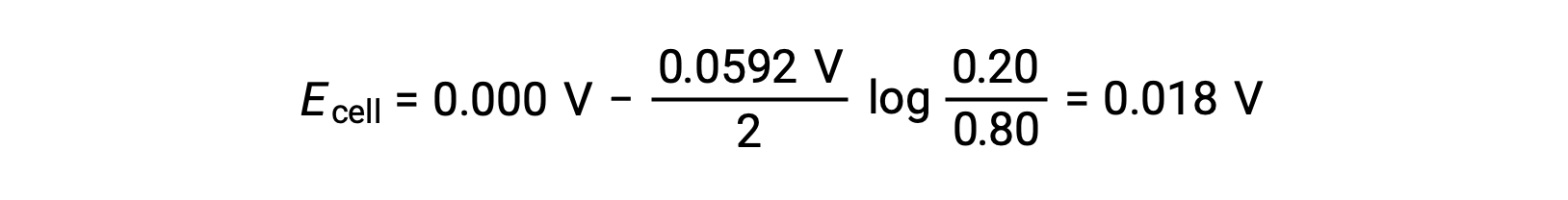
電極電位の値が正であることは、電池全体の反応が自発的であることを示しています。この自発的な反応は、カソードの亜鉛イオン濃度が下がり(亜鉛単体に還元される)、アノードの亜鉛イオン濃度が上がる(陽極の亜鉛が亜鉛イオンに酸化される)ことで起こります。Zn2+イオン濃度が高いカソードでは、亜鉛の還元に大きな駆動力が働く(Ecathode > Eanode)。
実験室のpHメーター、神経細胞膜のイオンチャンネル、人体の心筋細胞などは、濃淡電池の原理で動いています。
上記の文章は以下から引用しました。 Openstax,Chemistry 2e,Chapter 17.4: Potential, Free Energy, and Equilibrium.
Tags
Concentration CellsCell PotentialRedox ReactionReactantsProductsNickel-silver Galvanic CellStandard ConditionsConcentration GradientElectrochemical CellHalf-reactionsSilver ElectrodesSilver IonsOxidationReductionLe Châtelier’s PrincipleElectron FlowDilute CellConcentrated CellNernst EquationEquilibriumPH Meters
