U の尿素の内転によって分岐、環状化合物の除去k'37 Paleothermometry
English
Share
Overview
ソース: ジェフ Salacup – マサチューセッツ大学アマースト校講座
以前の動画と有機溶媒抽出、製品に記載されている総脂質抽出物 (TLE) は多くの場合、さまざまな化合物の何千人もいない場合、何百もの複雑な混合物。研究者は頻繁にただ化合物の一握りに興味があります。私たちの 2 つの有機 paleothermometers の場合 (Uk’37と MBT/CBT)、関心は (2 アルケノンフラックスおよび 4 isoprenoidal グリセロール ジアルキル グリセロール tetraethers) のみ 6 化合物で。このシリーズの前の 2 つのビデオで説明したように、分析サンプル中の化合物の数を削減するために浄化技術を適用できます。これらの技術が化学的に不要なコンポーネント (鹸化) を変更、異なる化合物の化学的性質 (クロマトグラフィー) の活用または異なった形および分子のサイズを使用して、または解析 (尿素転) から特定のコンポーネントを除外します。異なる化学物質の原子構造をリードする狭い長いを形成するいくつかの有機化合物、直鎖 (n-アルカンおよびアルケノン) 複雑な繰返し構造を形成する、他の高分岐構造を形成、その他の有機化合物とまだ他は両方の繰返しを形成して分岐構造 (GDGTs) (図 1)。異なった形およびサイズのサンプル中の化合物は、コイン選別機を分けるさまざまな宗派 (サイズ) の硬貨とほぼ同じ方法で、互いからそれらを分離する使用ことができます。
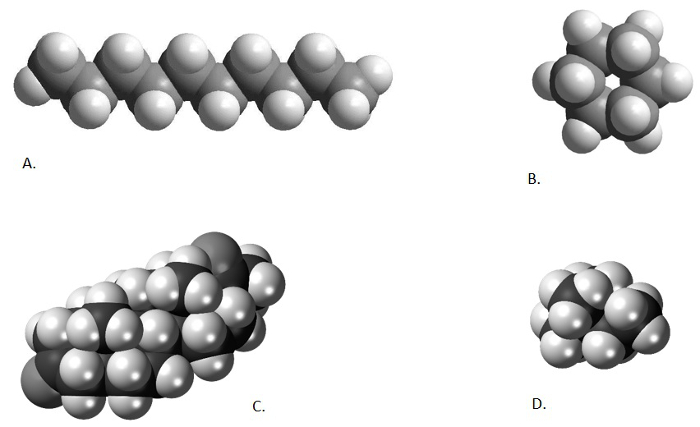
図 1。異なる化学構造体の比較します。デカン、直鎖アルカン (A; から http://www.bpc.edu/mathscience/chemistry から)、シクロヘキサン、環状アルカン (B; http://www.bpc.edu/mathscience/chemistry から)、ステロイド、多環炭化水素 (C; www.wikiwand.com から)、および 2, 2-ジメチル ブタン、枝分かれしたアルカン (D; www.wikimedia.com)。この図の拡大版を表示するのにはここをクリックしてください。
Principles
Procedure
Results
This purification technique produces two different vials; one labeled adduct, containing straight-chained and rarely-branching compounds, and another labeled non-adduct, containing highly-branched and cyclic compounds. This procedure has vastly decreased the complexity of any sample to be analyzed on an instrument. This decrease in complexity is often crucial to the accurate analysis of target compounds. For example, in nearshore settings after approximately 1850, alkenones co-elute with troublesome compounds, ostensibly pollutants introduced after the Industrial Revolution, which are not removed using column chromatography or saponification alone. Apparently, the pollutants are cyclic or branched because urea adduction removes them from the sample. The top 160 years of some of these sediment archives can be confidently analyzed for Uk'37 only because of the application of urea adduction.
Applications and Summary
Urea adduction is often used in the purification of n-alkanes, common constituents of leaf wax, in order to remove co-eluting compounds before isotope analysis. The carbon and hydrogen isotope ratios of leaf waxes in plants contain information on the metabolic pathways and environmental conditions the plant used and lived in, respectively. In order to determine the isotope ratios, very large quantities of compound must be loaded onto a GC. Such large quantities often cause compounds that elute close to one another at lower concentrations to co-elute. Often, the compounds co-eluting with alkanes are branched or cyclic moieties of that alkane and can thus be removed using urea adduction.
Transcript
Urea adduction is a technique that separates straight-chained alkenones from highly-branched and cyclic compounds.
Urea is an organic compound that forms a porous crystalline structure. The crystal can trap certain molecules, forming an adduct, while others can’t fit within the structure.
Urea adduction utilizes the different sizes and shapes of compounds to separate them – similar to how a coin counting machine will sort a jar of loose change.
This video is part of a series on lipid extraction, purification, and analysis from sediments. It will illustrate the use of urea adduction to perform size exclusion purification on a sample for alkenone paleothermometry.
Urea adduction is a purification method based on size exclusion. It separates straight-chained or rarely-branching compounds – such as n-alkanes and alkenones – from those that are highly-branched and/or cyclic.
This is possible because of urea’s special crystalline structure. When a urea crystal forms, tiny spaces are created between the individual molecules. These spaces are long and narrow – so straight-chain or rarely-branching compounds can fit into the crystal lattice, while highly-branched and cyclic compounds are too large.
The urea crystals are then washed with an apolar solvent, separating the excluded molecules from the included linear ones. The washed molecules can be extracted and analyzed directly, while the crystals must be dissolved in water to release the linear compounds back into solution first. Another apolar solvent is then used to extract the desired compounds from the water.
Both the included and excluded molecules can provide valuable information. For example, highly branched isoprenoids, produced by sea ice diatoms, can be a proxy for the existence of seasonal sea ice at high latitudes. Cyclic compounds may reflect the presence of past fires. Straight chained alkanes and alkenones are common proxies for ecosystem structure and sea surface temperature.
The purification provided by urea adduction is not always necessary, as some compounds of interest can be analyzed directly from the unaltered extracted organic sample. In extreme cases – such as in sediments acquired from highly polluted areas, like estuaries near industrial centers – a urea adduction may be necessary to remove unknown compounds that coelute during analysis.
Now that you understand urea adduction, you are ready to begin the procedure.
To begin, acquire a dried total lipid extract – or TLE – that has been extracted from the sample and purified with saponification and column chromatography.
Next, prepare the urea adduction solutions as outlined in the text protocol. Ensure that all components are pure and free of hydrocarbons.
Suspend the sample in 1.5 mL of the DCM/hexane solution. If the TLE does not completely dissolve, sonicate for 5 min. Add 1.5 mL of the urea and methanol solution. Watch for the formation of a white precipitate, as this signals the creation of urea crystals. Next, gently dry the urea crystals under nitrogen, using gentle heat. Be sure to evaporate all of the solvent. Once the crystals are completely dried, rinse them 3 times with approximately 1 mL of hexane. Use a glass pipette to remove the hexane between each rinse, and transfer it to a fresh vial. This is labeled the “non-adduct”. Next, dissolve the crystals in 2 mL of pure water. Shake to ensure complete dissolution.
To extract the biomarkers from the added water, add 1 mL of hexane, cap, and gently shake for 5 s.
Allow the solution to rest until the hexane and water separate completely. Then, using a glass pipette, remove approximately 75% of the hexane and transfer it into a new vial. This is labeled the “adduct”.
Repeat this extraction process twice, adding 1 mL of hexane each time. Combine the three adducts into one vial. Dispose of the water and urea solution in an appropriate waste container. The sample is now ready for analysis.
Urea adduction has several applications in the separation and purification of organic molecules.
An isotope is a variant of a chemical element that differs in the neutron quantity, and thus differs in its atomic mass. An element may have several isotopes, each having a different mass. Isotopes can also have chemical and molecular properties that differ from one another, so it can be important to determine which isotopes are present in a particular sample. In this example, the carbon and hydrogen isotope ratios in leaf waxes were measured in order to gather information on a plant’s metabolic pathways. Very large quantities of material are required to determine isotope ratios, and compounds that have similar detections at low concentrations may overlap when large quantities are analyzed.
Therefore, urea adduction was used to separate the n-alkanes of interest from any interfering compounds. Removing these unwanted materials allowed for an accurate isotope ratio to be determined.
Petroleum is a complex mixture of hydrocarbons, each with unique properties and uses. The separation of these compounds from petroleum is very important in the chemical industry – branched-chain alkanes are often used as light lubricants, while straight-chain alkanes are mainly used in alkylation processes. In this example, urea adduction was used to separate alkanes from kerosene. Using a successive series of urea adductions, these alkanes were separated from kerosene at a 99-percentage purity.
You’ve just watched JoVE’s introduction to the purification of complex organic mixtures via urea adduction. You should now understand size exclusion, the importance of purifying samples for accurate component measurement, and urea adduction.
Thanks for watching!
