Uk'37 고수온계의 요소 내전에 의한 분지 및 고리 화합물의 제거
English
Share
Overview
출처: 제프 살라컵 연구소 – 매사추세츠 대학교 애머스트
이전 비디오에서 언급했듯이, 유기 용매 추출의 산물, 총 지질 추출물 (TLE)은 종종 수백, 수천 이 아니라면 다른 화합물의 복잡한 혼합물입니다. 연구원은 종종 화합물의 소수에 만 관심이. 우리의 두 유기 고생물학 (Uk’37 및 MBT/CBT)의 경우, 관심은 단지 6 화합물 (2 알케네톤 과 4 isoprenoidal 글리세롤 글리세롤 글리세롤 테트라에테르)에 있습니다. 본 계열에서 이전 두 개의 비디오에서 설명한 바와 같이, 정제 기술은 분석된 샘플에서 화합물의 수를 줄이도록 하기 위해 적용될 수 있다. 이러한 기술은 화학적으로 원치 않는 구성 요소(saponification)를 변화시키고, 상이한 화합물 화학(컬로마그래피)을 활용하거나, 분자의 다양한 모양과 크기를 사용하여 특정 성분을 분석(urea adduction)에서 포함하거나 배제할 수 있다. 상이한 화학물질의 원자구조는 일부 유기 화합물을 형성하여 길고 좁은 직선 사슬(n-alkanes 및 alkenones), 다른 유기 화합물이 복잡한 순환 구조를 형성하고, 다른 화합물은 고도로 분진 된 구조를 형성하고, 또 다른 일부는 순환 및 분기 구조 (GDGTs)(도 1)를형성한다. 샘플내 화합물의 다양한 모양과 크기는 동전 선별기와 거의 같은 방식으로 서로 분리하는 데 사용할 수 있으며, 이는 다른 교단(크기)의 동전을 분리한다.
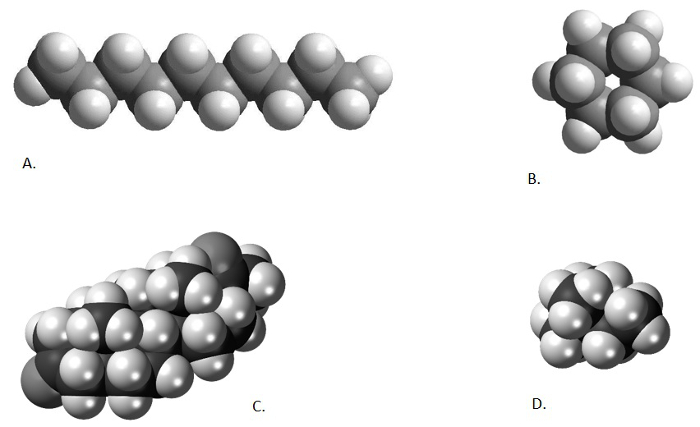
그림 1. 다른 화학 구조의 비교. Decane, 직선 체인 알케인 (A; http://www.bpc.edu/mathscience/chemistry), 사이클로 헥산, 순환 알케인 (b; http://www.bpc.edu/mathscience/chemistry), 스테로이드, 폴리 순환 탄화수소 (C; www.wikiwand.com), 그리고 2,2-디메틸부탄, 가지 알케인 (D; www.wikimedia.com). 이 그림의 더 큰 버전을 보려면 여기를 클릭하십시오.
Principles
Procedure
Results
This purification technique produces two different vials; one labeled adduct, containing straight-chained and rarely-branching compounds, and another labeled non-adduct, containing highly-branched and cyclic compounds. This procedure has vastly decreased the complexity of any sample to be analyzed on an instrument. This decrease in complexity is often crucial to the accurate analysis of target compounds. For example, in nearshore settings after approximately 1850, alkenones co-elute with troublesome compounds, ostensibly pollutants introduced after the Industrial Revolution, which are not removed using column chromatography or saponification alone. Apparently, the pollutants are cyclic or branched because urea adduction removes them from the sample. The top 160 years of some of these sediment archives can be confidently analyzed for Uk'37 only because of the application of urea adduction.
Applications and Summary
Urea adduction is often used in the purification of n-alkanes, common constituents of leaf wax, in order to remove co-eluting compounds before isotope analysis. The carbon and hydrogen isotope ratios of leaf waxes in plants contain information on the metabolic pathways and environmental conditions the plant used and lived in, respectively. In order to determine the isotope ratios, very large quantities of compound must be loaded onto a GC. Such large quantities often cause compounds that elute close to one another at lower concentrations to co-elute. Often, the compounds co-eluting with alkanes are branched or cyclic moieties of that alkane and can thus be removed using urea adduction.
Transcript
Urea adduction is a technique that separates straight-chained alkenones from highly-branched and cyclic compounds.
Urea is an organic compound that forms a porous crystalline structure. The crystal can trap certain molecules, forming an adduct, while others can’t fit within the structure.
Urea adduction utilizes the different sizes and shapes of compounds to separate them – similar to how a coin counting machine will sort a jar of loose change.
This video is part of a series on lipid extraction, purification, and analysis from sediments. It will illustrate the use of urea adduction to perform size exclusion purification on a sample for alkenone paleothermometry.
Urea adduction is a purification method based on size exclusion. It separates straight-chained or rarely-branching compounds – such as n-alkanes and alkenones – from those that are highly-branched and/or cyclic.
This is possible because of urea’s special crystalline structure. When a urea crystal forms, tiny spaces are created between the individual molecules. These spaces are long and narrow – so straight-chain or rarely-branching compounds can fit into the crystal lattice, while highly-branched and cyclic compounds are too large.
The urea crystals are then washed with an apolar solvent, separating the excluded molecules from the included linear ones. The washed molecules can be extracted and analyzed directly, while the crystals must be dissolved in water to release the linear compounds back into solution first. Another apolar solvent is then used to extract the desired compounds from the water.
Both the included and excluded molecules can provide valuable information. For example, highly branched isoprenoids, produced by sea ice diatoms, can be a proxy for the existence of seasonal sea ice at high latitudes. Cyclic compounds may reflect the presence of past fires. Straight chained alkanes and alkenones are common proxies for ecosystem structure and sea surface temperature.
The purification provided by urea adduction is not always necessary, as some compounds of interest can be analyzed directly from the unaltered extracted organic sample. In extreme cases – such as in sediments acquired from highly polluted areas, like estuaries near industrial centers – a urea adduction may be necessary to remove unknown compounds that coelute during analysis.
Now that you understand urea adduction, you are ready to begin the procedure.
To begin, acquire a dried total lipid extract – or TLE – that has been extracted from the sample and purified with saponification and column chromatography.
Next, prepare the urea adduction solutions as outlined in the text protocol. Ensure that all components are pure and free of hydrocarbons.
Suspend the sample in 1.5 mL of the DCM/hexane solution. If the TLE does not completely dissolve, sonicate for 5 min. Add 1.5 mL of the urea and methanol solution. Watch for the formation of a white precipitate, as this signals the creation of urea crystals. Next, gently dry the urea crystals under nitrogen, using gentle heat. Be sure to evaporate all of the solvent. Once the crystals are completely dried, rinse them 3 times with approximately 1 mL of hexane. Use a glass pipette to remove the hexane between each rinse, and transfer it to a fresh vial. This is labeled the “non-adduct”. Next, dissolve the crystals in 2 mL of pure water. Shake to ensure complete dissolution.
To extract the biomarkers from the added water, add 1 mL of hexane, cap, and gently shake for 5 s.
Allow the solution to rest until the hexane and water separate completely. Then, using a glass pipette, remove approximately 75% of the hexane and transfer it into a new vial. This is labeled the “adduct”.
Repeat this extraction process twice, adding 1 mL of hexane each time. Combine the three adducts into one vial. Dispose of the water and urea solution in an appropriate waste container. The sample is now ready for analysis.
Urea adduction has several applications in the separation and purification of organic molecules.
An isotope is a variant of a chemical element that differs in the neutron quantity, and thus differs in its atomic mass. An element may have several isotopes, each having a different mass. Isotopes can also have chemical and molecular properties that differ from one another, so it can be important to determine which isotopes are present in a particular sample. In this example, the carbon and hydrogen isotope ratios in leaf waxes were measured in order to gather information on a plant’s metabolic pathways. Very large quantities of material are required to determine isotope ratios, and compounds that have similar detections at low concentrations may overlap when large quantities are analyzed.
Therefore, urea adduction was used to separate the n-alkanes of interest from any interfering compounds. Removing these unwanted materials allowed for an accurate isotope ratio to be determined.
Petroleum is a complex mixture of hydrocarbons, each with unique properties and uses. The separation of these compounds from petroleum is very important in the chemical industry – branched-chain alkanes are often used as light lubricants, while straight-chain alkanes are mainly used in alkylation processes. In this example, urea adduction was used to separate alkanes from kerosene. Using a successive series of urea adductions, these alkanes were separated from kerosene at a 99-percentage purity.
You’ve just watched JoVE’s introduction to the purification of complex organic mixtures via urea adduction. You should now understand size exclusion, the importance of purifying samples for accurate component measurement, and urea adduction.
Thanks for watching!
