הפעלת כלי כור בלחץ גבוה
English
Share
Overview
רוברט מ. ריו, אוניברסיטת פנסילבניה, פארק האוניברסיטאות, הרשות הפלסטינית
השימוש בגזים במעבדה לכימיה סינתטית חיוני לביצוע מגוון של טרנספורמציות חסכוניות ואטום. תגובות כגון הידרוגנציה, חמצון ומינוי דורשות שימוש בגזים כמו מימן, חמצן ואמוניה. בשל המסיסות הירודה של גזים אלה בפתרונות מגיבים טיפוסיים, לחצים גבוהים נחוצים כדי להשיג שיעור תגובה משמעותי. לא רק גזים אלה מגיבים מאוד, השימוש בלחצים גבוהים הופך את הפעולות האלה למסוכנות למדי. האתגר הגדול ביותר בשימוש בלחץ גבוה הוא בלימת הגז בלחץ גבוה למשך כל התגובה, עם ניטור צמוד של הלחץ והטמפרטורה, כדי למנוע היווצרות של תערובות נפץ ותגובות בורחות.
תגובות אלה מתבצעות בדרך כלל באמצעות כלי לחץ עם קירות עבים. כלי השיט בלחץ מאפשר פעולה בלחץ גבוה עם חששות בטיחות מתאימים דעכו. איור 1 מדגים את החלקים השונים של כלי לחץ טיפוסי, המשמשים לביצוע תגובות בלחץ גבוה. הפרוטוקול הבא מדגיש את ההליך להפעלה בטוחה של כלי כור בלחץ גבוה אלה.
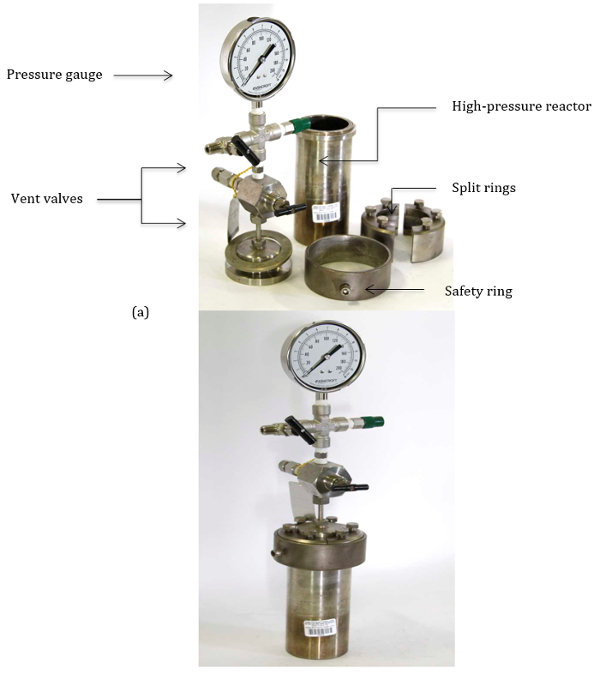
איור 1. (א) חלקים מכלי הכור בלחץ גבוה. (ב) כלי כור מורכב בלחץ גבוה.
Procedure
Applications and Summary
The manipulation of gases at high pressure can be done with the use of a Parr reactor (or equivalent) vessel. Proper safety precautions should be observed while operating these vessels as they present an explosion hazard.
Transcript
The use of gases in the synthetic chemistry laboratory is essential for carrying out a variety of highly facile and atom economical transformations, and often require high pressures to ensure sufficient solubility of gases into the reactant solution.
Reactions such as hydrogenation, oxidation, and amination require the use of gases like hydrogen, oxygen, and ammonia, respectively. Due to the poor solubility of these gases in typical reactant solutions, high pressures are necessary to achieve a meaningful reaction rate. Therefore, high-pressure reactor vessels – thick-walled containers, typically made of stainless steel – are used to carry out such reactions. The pressurized vessel allows for operation at high pressure with appropriate safety concerns abated.
In this video, we will first review the safety considerations and then learn how to charge, purge, and vent a high-pressure reactor vessel.
High-pressure reactor vessels can maintain environments of 3,000 PSI and 500 degrees. Vessels rated for higher pressures require thicker walls, though, making temperature control more difficult.
The manufacturer’s limits must be maintained during operation, as the gases are highly reactive, as well as the high pressure being a hazard itself. In addition to temperature and pressure, capacity and corrosion resistance must also be kept in mind when setting up an experiment.
The reaction itself must also be considered, as some reactions, like Hydroformylation, produce heat or while others like the Haber-Bosch-Process result in gaseous products. Too much heat or gas formation could push the reactor outside its operating limits leading to an explosion.
With these safety considerations in mind, let’s see how to work with these vessels.
To begin the procedure, select a clean secondary vessel in which the reaction will take place. Depending on the reaction’s scale, this can be a test tube, Erlenmeyer, or round-bottomed flask.
Add the reactants along with a clean stirbar into the secondary vessel.
Place the pressure gauge assembly on top of the reaction vessel. Close the vent valve by turning it clockwise until finger tight.
Add the split rings onto the vessel, tightening diagonally opposite screws to seal the reactor. Do not tighten the screws all at once to ensure even pressure across the vessel.
Place the reactor inside the safety ring on the benchtop. Slide the ring over the split rings, and align the screw with the indent on the split ring.
Finger tighten the safety ring. With the reactor sealed, it is ready to be purged and pressurized.
The next step is to purge the affixed reactor with an inert gas. Attach the gas source to the reactor and open the main valve on the regulator.
Using the cylinder regulator set the pressure to approximately 1/3rd of the final required value. Slowly open the vent valve on the pressure gauge and pressurize the reactor.
When desired pressure is reached, close the valve to the autoclave, followed by the valve to the gas source on the regulator and the cylinder valve.
Slowly loosen the pressure line going into the reactor, so that the pressure in the reactor starts to fall. Once the pressure falls back to zero, close the pressure line again and open the main valve on the regulator to the gas source.
Repeat the previous process with 2/3rd of the final pressure.
Now adjust the pressure on the regulator to the final desired value and pressurize the reactor. Once the final pressure is reached, close the vent valve on the pressure gauge, and close the main valve on the gas regulator.
Carefully loosen the pressure line, so that the gas in the line and the regulator is vented. This ensures that the gas source is disconnected from the reactor, which is important, once chemistry has been initiated.
Set the outlet pressure on the cylinder regulator back to zero by loosening the pressure control valve. This ensures that gas will not leak, even if the main valve on the regulator is turned on by accident.
Now place the reactor in a fume hood and let the reaction run for the desired amount of time. The reactor can be heated if desired.
The next step is to safely vent the completed reaction. Once the reaction time has elapsed, cool the reactor to room temperature.
Then, slowly open the vent valve on the gauge to vent the gas from the reactor. Do this as slow as possible to avoid the solvent from spilling over in the reactor.
Once the pressure in the reactor has dropped to zero, loosen the safety ring and the screws on the split rings. Disassemble the split rings and remove the gauge from the reactor.
Collect the reaction vessel from the reactor. Rinse the reactor with water and the acetone. Leave it open to air dry.
You’ve just watched JoVE’s introduction to using high-pressure reactor vessels. You should now understand their function, and how to properly charge, pressurize, and vent one. Thanks for watching!
