העברת תאים מאמצת: הצגת טחול עכבר תורם לעכבר מארח והערכה של הצלחה באמצעות FACS
English
Share
Overview
מקור: מונירסילבן 1,2,3, פרצ’טתיבו 1,2,3, סופינובולט 4, רחל גולוב1,2,3
יחידה אחת ללימפופוליס, המחלקה לאימונולוגיה, מכון פסטר, פריז, צרפת
2 INSERM U1223, פריז, צרפת
3 100é Paris Diderot, סורבון פריז סיטה, צ’רול פסטר, פריז, צרפת
4 פלטפרום ציטומטריה זרימה, ציטומטריה וסמנים ביולוגיים UtechS, המרכז למדע תרגום, מכון פסטר, פריז, צרפת
העברת תאים מאמצת היא שיטה להכנסת תאים לחולה או לאורגניזם מחקרי על מנת לטפל במחלה או ללמוד תהליך ביולוגי, כגון המטופיס. מטרות ההעברה המאמצת שונות; זה יכול לשמש בביולוגיה בסיסית, כמו גם במדעי הרפואה (1, 2). במודלים של עכבר, העברה והפצה של תאים שהועברו ניתן ללמוד ואחריו מערכת מעקב (סמן משטח תא, כתמים על ידי CFSE, וכו ‘). במחקרי סרטן על מודלים עכבר, העברה של אוכלוסיות תאים ספציפיות יכולה לשמש כטיפול ניסיוני נגד גידולים. דוגמה נוספת לטכניקה זו היא יצירת עכברים כימריים על ידי העברת תאי מח עצם לעכברים או עכברים מוקרן עם פנוטיפ חיסוני חמור. מודל עכבר זה יכול לשמש כדי להעריך את ההשפעה של מחיקת גנים על אוכלוסיית תאים ספציפית למשל. העברת תאי השאלת עצם משמשת גם בטיפול רפואי אנושי. כאשר חולים מוקרנים במקרה של טיפול בסרטן, העברה מאמצת של מח עצם מאפשרת שחזור מערכת החיסון.
הצעד הראשון בטכניקה זו הוא להשיג את אוכלוסיית התאים של עניין. הטכניקה שנבחרה לבודד אוכלוסייה זו תלויה ברמת הספציפיות של האוכלוסייה הממוקדת. רמת הבחירה הגדולה ביותר היא האיבר כולו, שבו נלקחות כל אוכלוסיות התאים הקיימות באיבר. שיטה מדויקת יותר היא בחירה של אוכלוסיית תאי יעד, שנבחרה לעתים קרובות על-ידי סמן משטח תא אחד. השיטה האידיאלית למיין תאים במקרה זה היא על ידי מיון מגנטי. לבסוף, הרמה המחמירה ביותר היא בחירת התאים על ידי מספר סמני פני השטח של התא כדי למיין אוכלוסיות תאים ספציפיות מאוד. מיון ציטומטריית זרימה היא השיטה הפופולרית ביותר עבור רמה זו של בחירה. לאחר קבלת אוכלוסיית העניין, ניתן להעביר אותה למארח. לפני העברה מאמצת זה חיוני כדי להבטיח תאימות בין המארח לתורם. ואכן, ללא קשר למטרת ההעברה, התאימות חיונית כדי להבטיח אימוץ תאים על ידי המארח ללא דחיית תאים.
בתרגיל מעבדה זה, אנו מדגימים את טכניקת העברת התאים המאמצת על ידי העברת טחול מעכבר CD45.2 לעכבר CD45.1 Ragγc (חסר לימפוציטים) וארבעה ימים לאחר מכן מאשרים את העברת הטחול באמצעות ציטומטריית זרימה (ראה איור 1).
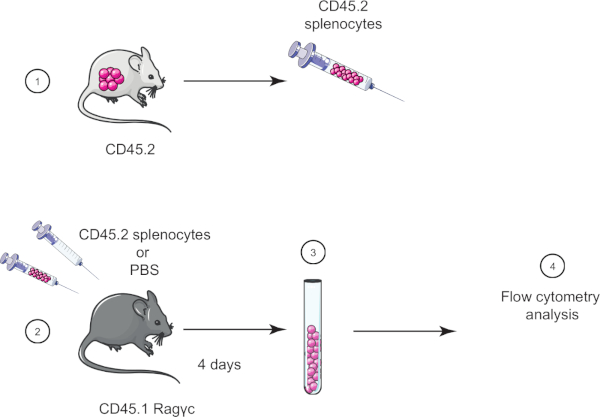
איור 1: ייצוג סכמטי של העברה מאמצת. (1) ספלוציטים מבודדים מעכברי CD45.2 ו- (2) מועברים בעכבר CD45.1 Ragγc, עכבר בקרה מוזרק ל- PBS בלבד. (3) 4 ימים לאחר העברה מאמצת, טחול הם התאוששו מעכברים ו (4) מנותח על ידי cytometry זרימה. אנא לחץ כאן כדי להציג גירסה גדולה יותר של איור זה.
Procedure
Results
Ragγc mice have an altered immune system composition, mainly lacking lymphocytes. Adoptive transfer of splenocytes allows introduction of lacking population such as T and B cells. Our staining included cell surface markers CD45.1 and CD45.2 to distinguish host and donor cells respectively (Figure 2A). It also included other cell surface marker to highlight cell populations absent in Ragγc mice, such as CD4 T cells (Figure 2B). As expected, control mouse did not have CD45.2-positive cells (Figure 2B, top panels) and transferred mouse did (Figure 2B, bottom panels, 71.2% of total cells). We could also specifically detect CD4 T cells within transferred cells (22.1% of CD45.2 cells).

Figure 2: Representative results of adoptive transfer. (A) Histograms of CD45.2 cells from mice injected with PBS (control group) (dashed) and mice injected with CD45.2 splenocytes (test group) (solid line). (B) Gating strategy of CD45.2-positive cells in control mice injected with PBS (top panels) and mice injected with CD45.2 splenocytes (bottom panels). Donor and host cells are distinguished using cell surface markers (CD45.1, CD45.2), then CD45.2-positive cell population are characterized (CD3, CD4). Please click here to view a larger version of this figure.
Applications and Summary
Adoptive transfer is a translational technique in different fields of science, with applications in medicine. This technique can be used to study cell migration and tropism or incidence of protein deficiency in specific cell populations. In the last case, different technologies can be used, especially GMO mice where specific cell populations are intrinsically deficient. However, genetic construction to obtain GMO mice can be a very complex and long process. In this case, adoptive transfer of deficient cell population is easier and faster.
Adoptive transfers have direct applications in medicine. For instance, bone marrow grafts in irradiated patients during cancer therapy are used to reconstitute the immune system. Recently, other applications of adoptive transfer have been used in the medical field. Artificial T cells (called CAR T cell) are designed to recognize and eliminate some cancers. In addition, these engineered cells are built to dampen rejection risk by the host. Transfer of CAR T cells is currently tested in clinical trials.
References
- Restifo, N. P., Dudley, M. E. and Rosenberg., S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews. Immunology, 12 (4): 269-281, (2012).
- Bonini, C., and Mondino, A. Adoptive T-cell therapy for cancer: The era of engineered T cells. European journal of immunology, 45 (9): 2457-69, (2015).
Transcript
Adoptive cell transfer is a method for introducing cells of interest into an organism. It is a powerful technique to study various biological mechanisms, including the action of specific classes of immune cells. In addition, adoptive transfer is a promising novel treatment for numerous conditions, such as those requiring bone marrow transplants or cancer treatments where a patient’s own T-cells can be extracted, altered to recognize and destroy the cancerous cells, and then returned to the body to fight tumors.
In the laboratory, animal models are often used to study adoptive transfer. For example, CD45.1 Rag gamma-c knockout mice lack fundamental receptors for many cytokines, which are essential for normal differentiation of hematopoietic stem cells into lymphocytes. As a result, the knockout mice have a compromised lymphocyte development and do not have natural killer, or NK, cells, T-cells, or B-cells.
Adoptive transfer can be used to introduce the missing immune cells into these compromised mice, by first harvesting donor mouse tissue containing high concentrations of immune cells, such as the spleen. The tissue is then dissociated and a variety of spleen cells, including the immune cells, are isolated. Next, unwanted erythrocytes, or red blood cells, can be lysed via the addition of ammonium chloride potassium lysing buffer and the remaining white blood cells, or splenocytes, are then separated from the cell debris using centrifugation.
Finally, the purified splenocytes are injected into the immunocompromised mice, facilitating detailed studies of these cells’ functions. Several days later, the success of the adoptive immune cell transfer can be confirmed by first isolating and preparing the host splenoncytes in the same manner as the donor tissue. Then, these cells are stained using labeled antibodies against the donor immune cell markers so that they can be verified and sorted using FACS.
To begin, put on laboratory gloves and the appropriate protective equipment. Next, wash a pair of forceps and dissecting scissors first with a detergent and then with 70% ethanol and then dry them with a clean paper towel. Prepare 50 milliliters of Hank’s Balanced Salt Solution, or HBSS, with 2% Fetal Calf Serum, or FCS, by combining one milliliter of FCS with 49 milliliters of HBSS in a 50 milliliter tube. Mix by gently pipetting the solution up and down approximately 10 times.
Dissect the euthanized mouse and remove its spleen as demonstrated in the JoVE video protocol FACS technology for splenic B lymphocytes separation. To isolate immune cells, first place the spleen on a 40 micrometer cell strainer in a petri dish. Crush the spleen with a plunger to dissociate it into the dish. Recover the adhered cells by rinsing the plunger and the strainer with 1 milliliter of HBSS 2% FCS. Then, pipette the dissociated spleen and fluid from the petri dish into a 50 milliliter centrifuge tube. Wash the petri dish with 5 milliliters of HBSS 2% FCS and transfer the fluid to the 15 milliliter tube.
Centrifuge the tube at 370 times g for seven minutes at 10 degrees Celsius and then retrieve the tube carefully so as not to disturb the pellet. Now, remove the supernatant without disturbing the pellet and discard the liquid in an appropriate waste container. Then, add two milliliters of ammonium chloride potassium lysing buffer to the centrifuge tube and pipette up and down to resuspend the pellet and lyse the erythrocytes. Wait for two minutes and then add HBSS 2% FCS to the resuspended pellet to obtain a total value of 14 milliliters. Repeat the centrifugation. Retrieve the tube carefully and discard the supernatant. Then, resuspend the pellet in 5 milliliters HBSS 2% FCS by pipetting up and down. Next, count the cells in suspension. Add five microliters of trypan blue to five microliters of cell suspension and mix well by pipetting. Then, gently deposit a five microliter drop of diluted cell suspension between the cover glass and the Malassez slide. With the microscope set to 40X magnification, count the number of cells. Adjust the cell concentration to 10 to the seven cells per milliliter by adding the appropriate volume of HBSS 2% FCS.
To begin the adoptive transfer, transfer two milliliters of the cell suspension to a five milliliter collection tube. Centrifuge the tube at 370 times g for seven minutes at 10 degrees Celsius and then discard the supernatant. Next, resuspend the pellet in two milliliters of phosphate buffered saline and centrifuge the tube at 370 times g for seven minutes at 10 degrees Celsius. Discard the supernatant. Then, resuspend the pellet in 200 microliters of phosphate buffered saline. Using a 0.5 milliliter syringe with a 29-gauge needle, inject 200 microliters of cell suspension into the experimental mouse intravenously into the retro-orbital blood sinus.
Four days after the adoptive transfer, euthanize the mice and remove the spleens. Then, harvest the immune cells as described in section three. Next, transfer 100 microliters of cell suspension from each mouse into two FACS tubes labeled control and transferred. Centrifuge the tubes at 370 times g for seven minutes at 10 degrees Celsius and then discard the supernatants. Now, prepare a mix containing the four antibodies at the dilution listed in table one. Add 100 microliters of the mix to each tube and then incubate for 20 minutes on ice in the dark. Next, add one milliliter of HBSS 2% FCS to each tube and then centrifuge the tubes at 370 times g for three minutes at 10 degrees Celsius. Discard the supernatants and then resuspend the pellets in 200 microliters of HBSS 2% FCS. Transfer the resuspended cells to new labeled FACS tubes. Now, use flow cytometry as shown in the FACS protocol to evaluate the presence of CD45. 2 positive lymphocytes.
Now, we will determine the presence CD45.2 lymphocytes that were isolated from the CD45. 1 host spleen. To start, double click on the FlowJo icon and drag the files for each tube in the all sample window. Then, double click on the transferred file to display the cells recorded from that sample on a dot plot that displays forward scatter FSCA on the x-axis and side scatter SSCA on the y-axis. Click on polygon to circle the lymphocyte populations. A new subpopulation identification window appears. Click on OK. Now, set the y-axis to FSC-W and the x-axis to FSC-A. Select the single cell population with the polygon tool as previously demonstrated.
Next, double click on the circled population to create a new window for the selected cells. In the new window, select CD45.2 on the Y and CD45.1 on the X. Click on the T icon and customize the axis to enlarge the plot. Next, click on polygon to circle the CD45.2 positive cells. In the subpopulation identification window, name your cell population transferred cells and click OK. In the same window, click on rectangle to select the CD45.2 negative cells. In the subpopulation identification window, name your cell population host cells and click OK. Next, double click on the CD45.2 circled population, transferred population, to create a new window for the selected cells. In the new window, select CD3 on the Y and CD4 on the X.
Next, click on polygon to circle the CD4 CD3 positive cells. In this subpopulation identification window, name your cell population transferred CD4 cells. Then, repeat the previous analysis steps with the control mouse file. Finally, to visualize your cell populations, click on Layout Editor. Drag the transferred cells and the transferred CD4 cells population from transferred and control files into the Layout Editor tab. A dot plot representing CD45.2 positive cells and CD4 lymphocytes will appear. CD45. 2 transferred cells should only appear in the transferred mouse dot plot.
