Cultures pures et ensemencement des géloses : isolement des colonies bactériennes pures à partir d'un échantillon mixte
English
Share
Overview
Source: Tilde Andersson1, Rolf Lood1
1 Département des sciences cliniques Lund, Division of Infection Medicine, Biomedical Center, Lund University, 221 00 Lund, Suède
Apparemment impossible à déterminer, la biodiversité microbienne est vraiment stupéfiante avec environ un billion d’espèces coexistantes (1,2). Bien que des climats particulièrement rudes, comme l’environnement acide de l’estomac humain (3) ou les lacs sous-glaciaires de l’Antarctique (4), puissent être dominés par une espèce spécifique, les bactéries se trouvent généralement dans les cultures mixtes. Comme chaque souche peut influencer la croissance d’une autre (5), la capacité de séparer et de cultiver des colonies « pures » (composées uniquement d’un seul type) est devenue essentielle dans les milieux cliniques et académiques. Les cultures pures permettent d’autres examens génétiques (6) et protéomiques (7), l’analyse de la pureté de l’échantillon et, peut-être plus remarquable, l’identification et la caractérisation des agents infectieux à partir d’échantillons cliniques.
Les bactéries ont un large éventail de besoins de croissance et il existe de nombreux types de supports nutritifs conçus pour soutenir à la fois les espèces non exigeantes et exigeantes (8). Les milieuis de croissance peuvent être préparés sous forme liquide (sous forme de bouillon) ou sous forme d’un agent gélifiant typiquement à base d’agar (un agent gélifiant dérivé d’algues rouges). Alors que l’inoculation directe dans le bouillon comporte le risque de générer une population bactérienne génétiquement diversifiée, voire mixte, le placage et le re-streaking crée une culture plus pure où chaque cellule a une composition génétique très similaire. La technique de la plaque de stries est basée sur la dilution progressive d’un échantillon (Figure 1), dans le but de séparer les cellules individuelles les unes des autres. Toute cellule viable (ci-après appelée unité de formation de colonie, CFU) soutenue par les médias et l’environnement désigné peut par la suite trouver une colonie isolée de cellules-filles par fission binaire. En dépit des taux rapides de mutation dans les communautés bactériennes, ce groupe de cellules est généralement considéré comme clonal. La récolte et le re-streaking de cette population assure par conséquent que les travaux ultérieurs ne concernent qu’un seul type bactérien.
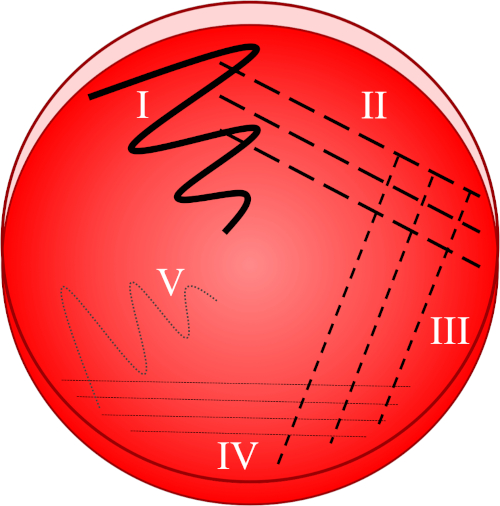
Figure 1 : Une plaque de stries est basée sur la dilution progressive de l’échantillon d’origine. I) L’inoculum est d’abord dispersé à l’aide d’un mouvement en zigzag, créant une zone avec une population bactérienne relativement dense. II-IV) Les stries sont tirées de la zone précédente, à l’aide d’une boucle d’inoculation stérile à chaque fois, jusqu’à ce que le quatrième quadrant soit atteint. V) Un dernier mouvement en zigzag dirigé vers le milieu de la plaque forme une région où l’inoculum a été nettement dilué, permettant aux colonies d’apparaître séparées les unes des autres.
La technique de la plaque de stries peut également être combinée à l’utilisation de supports sélectifs et/ou différentiels. Un milieu sélectif inhibera la croissance de certains organismes(p. ex. par l’ajout d’antibiotiques) tandis qu’un milieu différentiel aidera uniquement à distinguer l’un de l’autre(par exemple par hémolyse sur les plaques d’agar sanguine).
L’utilisation de techniques aseptiques (stériles) sous-tend tous les travaux en microbiologie. Chaque culture bactérienne devrait être considérée comme potentiellement pathogène car il existe un risque de croissance involontaire de souches dangereuses, la formation d’aérosols et la contamination de l’équipement /personnel. Pour minimiser ces risques, tous les aliments pour supports, plastiques, métalliques et vitraux sont généralement stérilisés par l’autoclacage avant et après l’utilisation, les soumettant à une vapeur saturée à haute pression à environ 121 oC qui élimine efficacement les cellules persistantes. L’espace de travail est généralement désinfecté à l’aide d’éthanol avant et après l’utilisation. Le manteau de laboratoire et les gants sont toujours portés pendant le travail avec des agents infectieux.
Procedure
Results
The initial streak-plate may contain colonies originating from cells with different genetic makeup or (depending on sample purity) from different bacterial species (Figure 2A).
Through subsequent isolation of a single colony, where all units are derived from a common mother-cell, the second streaking procedure generates a relatively clonal bacterial population, suitable for further characterization or inoculation into broth (Figure 2B).

Figure 2: A pure culture can be generated from a mixed sample through isolation of a single, secluded colony. A) Growth of a single bacterial cell (CFU) generated a clonal colony, separated from those of other species and strains. This CFU was used for subsequent streaking onto a new plate B) A second plate, where the bacterial population consists solely of cells derived from the initial CFU.
Applications and Summary
The ability to obtain and cultivate a pure bacterial colony is essential, both in clinical and academic settings. Streak plating enables the isolation of a relatively clonal cell population, originating from a shared CFU, that may be of particular interest during diagnosis or for additional characterization of the isolate. A sample is streaked onto a suitable agar-based nutrient medium and incubated until colonies become visible. An isolated colony is subsequently harvested and re-streaked onto a second plate.
References
- The Human Microbiome Project C. Structure, Function and Diversity of the Healthy Human Microbiome. Nature. 486:207-214. (2012)
- Locey KJ, Lennon JT. Scaling laws predict global microbial diversity. Proceedings of the National Academy of Sciences. 113 (21) 5970-5975 (2016)
- Skouloubris S, Thiberge JM, Labigne A, De Reuse H. The Helicobacter pylori UreI protein is not involved in urease activity but is essential for bacterial survival in vivo. Infection and Immunity. 66:4517-21. (1998)
- Mikucki JA, Auken E, Tulaczyk S, Virginia RA, Schamper C, Sørensen KI, Doran PT, Dugan H, Foley N. Deep groundwater and potential subsurface habitats beneath an Antarctic dry valley. Nature Communications. 6:6831. (2015)
- Mullineaux-Sanders C, Suez J, Elinav E, Frankel G. Sieving through gut models of colonization resistance. Nature Microbiology. 3:132-140. (2018)
- Fournier PE, Drancourt M, Raoult D. Bacterial genome sequencing and its use in infectious diseases. Lancet Infectious Diseases. 7:711-23 (2007)
- Yao Z, Li W, Lin Y, Wu Q, Yu F, Lin W, Lin X. Proteomic Analysis Reveals That Metabolic Flows Affect the Susceptibility of Aeromonas hydrophila to Antibiotics. Scientific Reports. 6:39413 (2016)
- Medina D, Walke JB, Gajewski Z, Becker MH, Swartwout MC, Belden LK. Culture Media and Individual Hosts Affect the Recovery of Culturable Bacterial Diversity from Amphibian Skin. Frontiers in Microbiology. 8:1574 (2017)
Transcript
On a Petri dish, if a single bacterium undergoes multiple rounds of asexual reproduction, it will lead to the formation of a clonal colony. However, obtaining a single bacterium from a mixed sample, such as a soil suspension, can be difficult. If one loopful of this heterogeneous culture is taken, it can contain as many as one trillion individual bacteria. To spread this many bacteria out onto the surface of an agar plate and obtain a single colony, even using a zig-zag pattern, the loop would need to be dragged continuously over the surface of enough plates set side-by-side to encircle the entirety of Liberty Island. Obviously, scientists do not really use that many plates. Instead, they use a technique called streak plating.
The streak plate technique is based on progressive dilution of a bacterial sample, and it is performed over the solid media surface of a single Petri dish. To begin, the media surface is visually divided into five sections by assigning four fragments of the circumference as the first four sections, and the plate’s center as the fifth. This will effectively create five media plates out of a single Petri dish. Next, using a loopful of desired inoculum, the first section is streaked using a zig-zag pattern. Then, either a new disposable loop is used, or in the case of a wire loop, it is sterilized with a Bunsen burner, flaming it until it is red hot along the length of the wire. This use of a new loop, or flame sterilize loop, removes any remaining bacterial cells, assisting in the dilution of the bacteria. The hot loop is then cooled in the air for a few seconds before being dragged through the first section to create three to four separate lines, each carrying only a fraction of bacteria into the second section. The remaining sections are streaked in the same manner, using a sterile loop each time, and a single pass through the previous streak.
Using this cycle of streaking and sterilizing, the bacterial concentration in every subsequent section should be diluted so that the final section contains only a few discretely located bacteria. Upon incubation, these discrete bacteria multiply to produce isolated clonal colonies of daughter cells, which are referred to as Colony Forming Units, or CFUs. These can be harvested and re-streaked to ensure that subsequent work involves only a single bacterial type, referred to as a pure culture. As well as isolating single colonies from a mixed-bacterial culture, the streak plating technique is also used to select media-specific strains, determine bacterial colony morphology, or identify different bacterial species. In this video, we will demonstrate how to isolate single-bacterial colonies from a mixed-bacterial sample suspension via streak plating technique.
To begin, put on laboratory gloves and a lab coat. Next, sterilize the workspace using 70% ethanol. Next, select a suitable medium that will sustain the utilized bacterial species or strain and begin preparing the media. Here, common LB agar is prepared by weighing out ten grams of pre-formulated, powdered media and 7.5 grams of agar. Add the weighed, dried components to a glass bottle which is able to hold twice the final volume to avoid overflow. Then, add 500 milliliters of water to the bottle, and cap it semi-tightly. Sterilize the media by placing the bottle in an autoclave set to 121 degrees Celsius for twenty minutes. After completion, use heat-proof gloves or a hot pad to remove the media from the machine and then immediately twist the bottle cap to close it tightly.
For the same-day use, let the media cool down by placing the bottle into a water bath heated to approximately 45 degrees Celsius, to preserve the media in a liquid state. Alternatively, the media can be left at room temperature to store at solid state. When needed, microwave the bottle with the lid slightly open to melt the media, and allow the media to cool using a 45 degree Celsius water bath.
Next, take a sleeve of sterile Petri dishes, and with a permanent marker, label them with the investigator and media names as well as the date. Then, transfer the required volume of media into a sterile vessel, and add antibiotics or other sensitive components if necessary. Here, 50 milliliters of media is mixed with 100 microliters of Kanamycin for a final concentration of 25 micrograms per milliliter. Swirl the tube to ensure even distribution of the added components throughout the media. Slowly, so as to avoid bubble formation, pour 20 to 25 milliliters of approximately 45 degree Celsius culture medium into each of the plates. If bubbles or foam appear, swiftly remove using a regular pipette and a sterile tip. Then, immediately replace all lids to prevent contamination. Allow the agar to solidify at room temperature for at least two hours or overnight. Once solidified, store the culture plates upside down at four degrees Celsius to minimize condensation on the medium’s surface.
To streak the culture of choice, first take a clean culture plate and remove the lid. Working quickly, submerge a disposable, sterile loop into the desired inoculum and then immediately swab the loop over the first quadrant of the plate using a zig-zag motion. Replace the lid of the dish, discard the used inoculation loop, and then select a new sterile loop. Using the new loop, make three to four strokes crossing the original swab line radiating from the first quadrant, which should contain a relatively dense bacteria population into the second quadrant. Close the lid once more, and discard the loop. With a new loop, repeat this action again, but this time streaking from the second into the third quadrant. Then, with a new loop again, make another streak from the third into the fourth section of the plate. Finally, with a fresh loop, make one last stroke in a zig-zag pattern from the fourth quadrant towards the center of the plate. The bacterial prevalence will be lower in this area, ideally allowing individual colonies to be established from a single viable mother cell.
Replace the plate lid, and if appropriate for the bacterial species, seal the plate with para film to prevent airflow. Turn the culture plate upside down to prevent condensation drips, and then place at a suitable temperature for growth. Here, an incubator is set to 37 degrees Celsius. Allow the plate to incubate until bacterial colonies are visible. To generate a clonal bacterial population, select one discrete colony from this plate. Now, with the sterile loop, touch the target colony, and as before, make a streak in the first quadrant of a new plate. Continue to alternately sterilize the loop and streak the remaining quadrants of the plate as previously demonstrated, ending with the zig-zag to the center. Close the plate, and place it to incubate until discrete colonies form. Once these colonies are grown, they will typically represent pure clonal strains.
The initial streak plate may contain colonies originating from cells from different bacterial species or cells with different genetic makeup, depending upon the sample purity. Through subsequent isolation of a single colony, where all units are derived from a common mother cell, the second streaking procedure generates a relatively clonal bacterial population, suitable for further characterization or inoculation into broth.
