18.10:
Electrolysis
18.10:
Electrolysis
In a galvanic cell, the electrical work is done by a redox system on its surroundings as electrons produced by the spontaneous redox reactions are transferred through an external circuit. Alternatively, an external circuit does work on a redox system by imposing a voltage sufficient to drive an otherwise nonspontaneous reaction in a process known as electrolysis. For instance, recharging a battery involves the use of an external power source to drive the spontaneous (discharge) cell reaction in the reverse direction, restoring to some extent the composition of the half-cells and the voltage of the battery. Other examples include the use of electrolysis in the refinement of metallic ores, the manufacture of commodity chemicals, and the electroplating of metallic coatings onto utensils, jewelry, etc.
Predicting the Product of Electrolysis
The electrolysis of molten sodium chloride, NaCl (l), is used for the industrial production of metallic sodium, Na, and chlorine gas, Cl2. Sodium ions (Na+) are reduced to atoms at the cathode, while chloride ions (Cl−) ions are oxidized to chlorine gas, Cl2, at the anode. The redox reactions are:
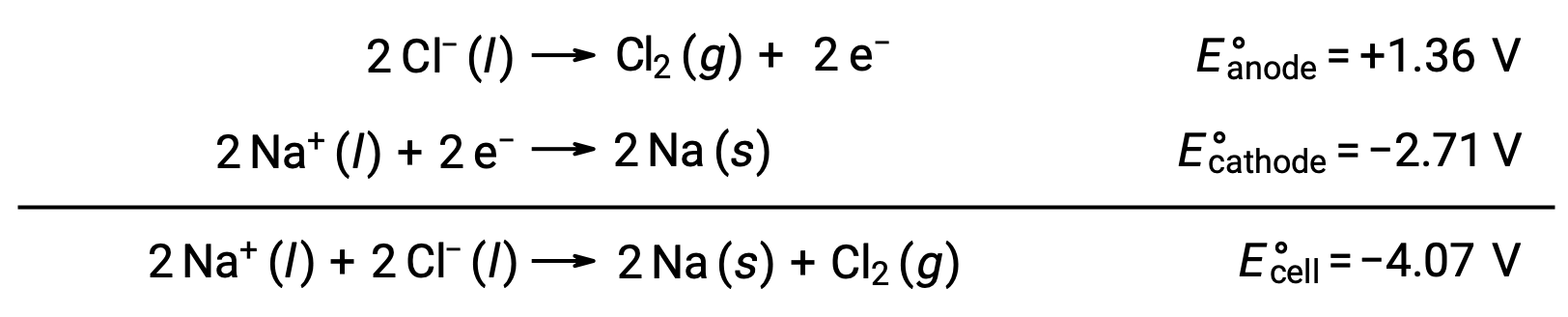
The negative cell potential indicates a nonspontaneous reaction that must be driven by imposing a positive potential of magnitude greater than −4.07 V. Electrolysis of molten sodium chloride is carried out at a high temperature of 801 °C as ionic solids melt at high temperatures.
The electrolysis of water produces stoichiometric amounts of oxygen gas at the anode and hydrogen at the cathode. To improve electrical conductivity, the hydrogen ion concentration of the water is increased by adding a strong acid. The associated redox reactions are:
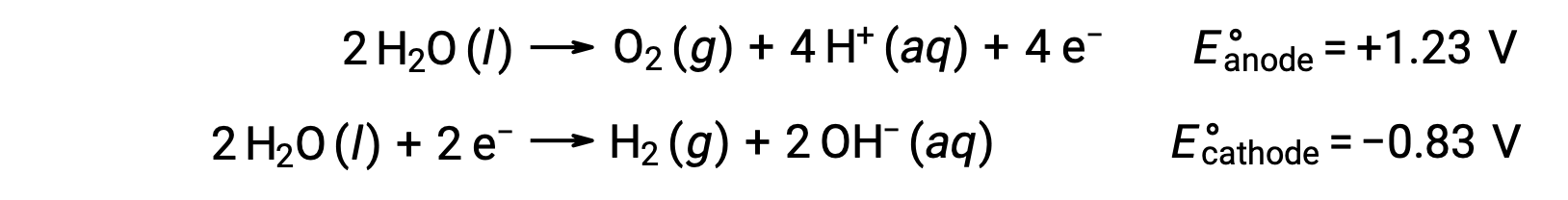
The electrolysis of solutions of ionic compounds such as aqueous sodium chloride may involve the electrolysis of either water species (H2O, H+, OH–) or solute species (the cations and anions of the compound) at the anode and cathode.
The electrolysis of aqueous sodium chloride could involve either of these two anode reactions:
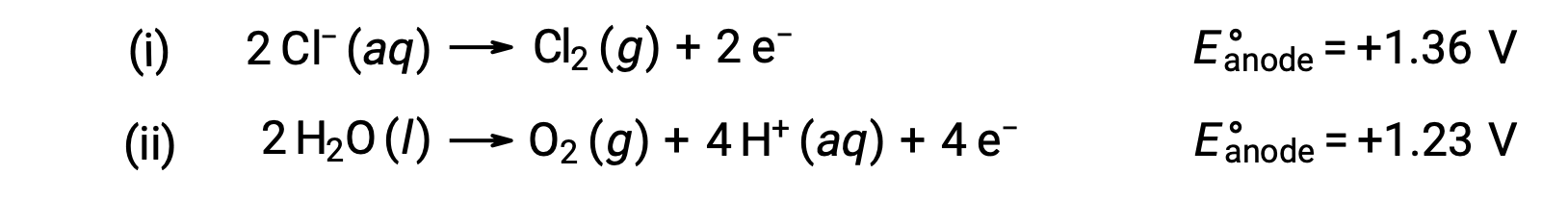
Thermodynamically, water would be more readily oxidized; however, in practice, chlorine gas is produced. Oxidation of water would require a much larger voltage to initiate. To overcome this overvoltage, electrodes are chosen and the potential of the cell is carefully monitored to ensure that the oxidation of chloride ions at the anode.
Similarly, the possible reduction reactions at the cathode are:
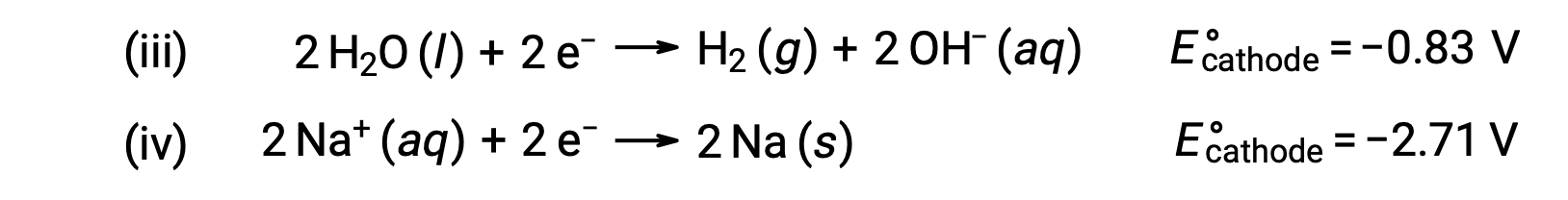
Comparing these standard half-reaction potentials suggests the reduction of water is thermodynamically favored. The net cell reaction in this case is then:
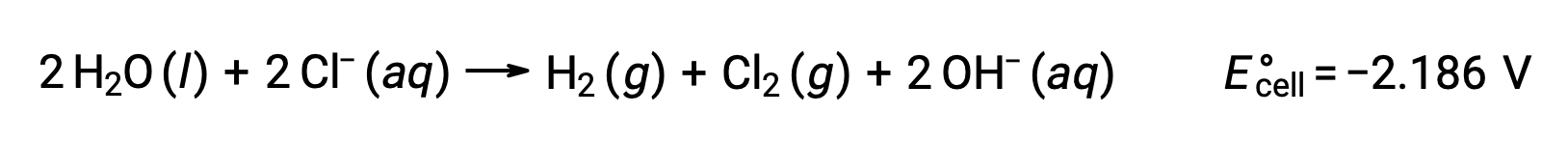
Quantitative Aspects of Electrolysis
Electrical current is the rate of flow of electrons and is measured in ampere, one coulomb per second (A = 1 C/s). The charge transferred, Q, by passage of a constant current, I, over a specified time interval, t, is given by
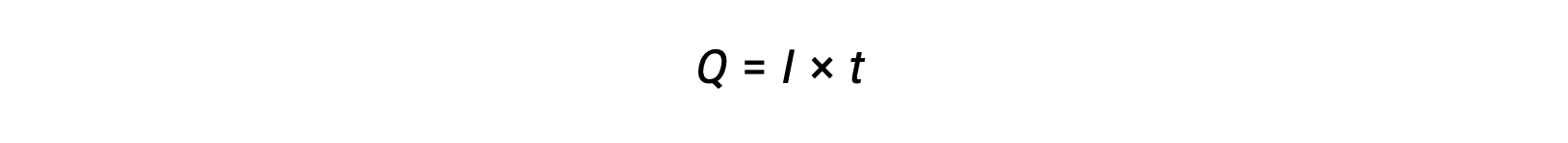
When electrons are transferred during a redox process, the stoichiometry of the reaction may be used to derive the total amount of (electronic) charge involved. For example, the generic reduction process,
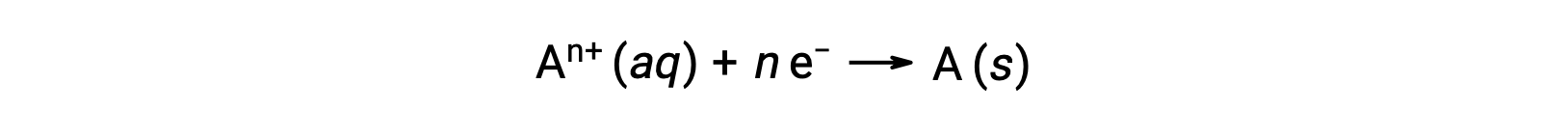
involves the transfer of n mole of electrons. The charge transferred is, therefore,
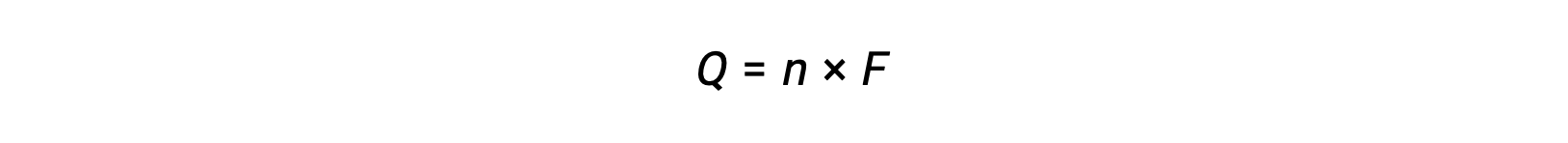
where F is Faraday’s constant, the charge in coulombs for one mole of electrons. For an electrochemical cell, the current flow is measured and can be used in stoichiometric calculations related to the cell reaction.
This text is adapted from OpenStax, Chemistry 2e, Section 17.7: Electrolysis.
