- 00:04Overview
- 01:03The Quadruple Metal-Metal Bond
- 03:49Synthesis of Ligand ArN(H)C(H)NAr (Ar = p-(MeO)C6H4)
- 05:09Synthesis of Molybdenum Paddlewheel Complex
- 06:40Single Crystal Growth
- 07:53Results
- 09:18Applications
- 10:41Summary
Enlance cuadruple de metal a metal en complejos de tipo rueda de paletas
English
Share
Overview
Fuente: Corey Burns, Tamara M. Powers, Departamento de química, Texas A & M University
Complejos de rueda son una clase de compuestos de dos iones del metal (1st, 2ndo metales de transición de fila de 3rd ) celebrados en proximidad de cuatro ligandos puente (más comúnmente formamidinates o carboxilatos) (figura 1). Variar la identidad de los iones metálicos y el ligando puente proporciona acceso a grandes familias de conjuntos de rueda. La estructura de los complejos de rueda permite vinculación de metal-metal, que desempeña un papel vital en la estructura y la reactividad de estos complejos. Debido a la diversidad de estructuras electrónicas que están disponibles para rueda complejos – y las correspondientes diferencias en el enlace de la M-M de estas estructuras – rueda complejos han encontrado aplicación en diversas áreas, tales como en homogéneo Catálisis y como bloques de construcción para el metal-organic frameworks (MOF). Comprender la estructura electrónica de los bonos M-M en rueda complejos es fundamental para entender sus estructuras y así a la aplicación de estos complejos de coordinación química y catálisis.
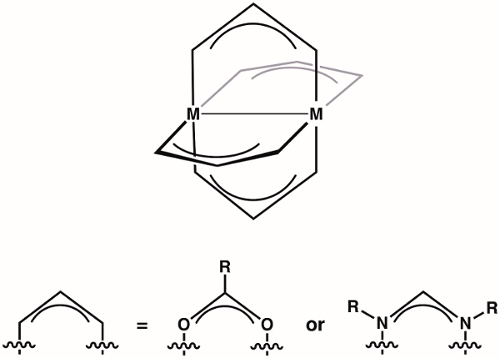
Figura 1. Estructura general de los complejos de la rueda, donde M puede ser 1st, 2ndo 3rd fila de metales de transición.
Cuando dos metales de transición se sitúan en las proximidades d-superposición de orbitales, que puede resultar en la formación de enlaces M-M. Superposición de d –orbitarios puede formar tres tipos de enlaces – σ, π y δ – dependiendo de la simetría de los orbitales involucrados. Si asignamos el eje molecular ser coplanares con el vínculo de M-M, un enlace σ está formado por el traslapo de los orbitarios de dz2 y enlaces π se forman por solapamiento de orbitales dyz los dxz . Δ los bonos son generados por la superposición de d-orbitarios que tienen dos nodos planares (dxy y dx2–y2). Como resultado, todos los cuatro lóbulos de la d-traslapo orbital y el correspondiente enlace δ tiene dos nodos planares (figura 2). En teoría, con la adición de enlaces δ, rueda complejos son capaces de soportar bonos quíntuples o cinco enlaces entre los átomos del metal. 1 en los más complejos, el dx2–y2 forma fuertes enlaces metal-ligando y no contribuye significativamente a M M Unión. Así, lazos cuádruples son del orden de máxima adherencia en muchos complejos.
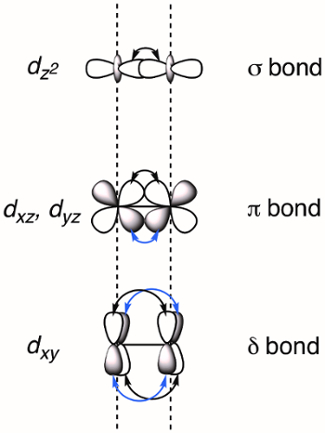
Figura 2. Representación visual de σ, π y δ vinculación MOs resultante de la combinación lineal de metal d-orbitarios. Los orbitales atómicos dz2 tienen el mejor solapamiento espacial, seguido del dxz y orbitales dyz . Los orbitales atómicos dxy tienen la menor cantidad de superposición espacial.
En este video, sintetizamos la dimolybdenum rueda complejo Mo2(ArNC(H)NAr)4, donde Ar = p-(MeO) C6H4, que cuenta con un enlace cuádruple. Vamos a caracterizar el compuesto por espectroscopia de RMN y usar Cristalografía de rayos x para estudiar el enlace M-M.
Principles
Procedure
Results
Ligand ArN(H)C(H)NAr
Yield: 3.25 g (53%). 1H NMR (chloroform-d, 500 MHz, δ, ppm): 8.06 (s, 1H, NHC-HN), 6.99 (d, 4H, aromatic C-H, J = 8.7 Hz), 6.86 (d, 4H, aromatic C-H, J = 9.0 Hz), 3.80 (s, 6H, -OCH3).
Mo complex Mo2(ArNC(H)NAr)4
Yield: 450 mg (57%). 1H NMR (chloroform-d, 500 MHz, δ, ppm): 8.38 (s, 4H, NHC-HN), 6.51 (d, 16H, aromatic C-H, J = 8.8 Hz), 6.16 (d, 16H, aromatic C-H, J = 8.8 Hz), 3.71 (s, 24H, -OCH3).
Table 1. Crystal Data and Unit Cell Parameters
| Empirical formula | C60H70Mo2N8O8 |
| Formula weight (g/mol) | 1223.12 |
| Temperature (K) | 296.15 |
| Crystal system | triclinic |
| Space group | P-1 |
| a (Å) | 10.1446(4) |
| b (Å) | 10.3351(4) |
| c (Å) | 13.9623(6) |
| α (°) | 80.151(2) |
| β (°) | 75.251(2) |
| γ (°) | 82.226(2) |
| Volume (Å3) | 1388.3(1) |
The 1H NMR spectrum of Mo2(ArNC(H)NAr)4 exhibits two signals in the aromatic region, which is consistent with 4-foldsymmetry. The solid-state structure (Figure 7) is consistent with the point group D4 and features a short Mo-Mo bond (2.0925(3) Å). The atomic radii of Mo are 1.45 Å. Therefore, using Equation 1, the FSR value for the M-M bond in Mo2(ArNC(H)NAr)4 is 0.72. This value is lower than that observed for the Mo-Mo quadruply bonded complex Mo(hpp)4 (hpp = 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidinate), which has an FSR value of 0.797, and is consistent with the presence of a M-M quadruple bond3.

Figure 7. Solid-state structure for Mo2(ArNC(H)NAr)4 with the thermal ellipsoids set at the 50% probability level. Hydrogen atoms are omitted for clarity (Mo navy, N blue, C gray).
Applications and Summary
In this video, we learned about M-M bonding. We synthesized a dinuclear molybdenum complex featuring a quadruple bond. Quadruple bonds consist of three different bond types, including σ, π, and δ bonds. We collected single crystal X-ray diffraction data and observed a short Mo-Mo bond length consistent with a quadruply bonded compound.
Paddlewheel complexes, such as the Mo2 complex prepared here, display a wide range of properties and thus find application in diverse areas of chemistry. For example, M-M bonds play an important role in catalysis: the dirhodium paddlewheel complex Rh2(OAc)4 is a known catalyst for C-H bond functionalization via carbene and nitrene transfer reactions (Figure 8). In a typical carbene transfer reaction, Rh2(OAc)4 reacts with a diazo compound to generate a Rh2 carbene intermediate. Subsequent insertion of the carbene into a C-H bond generates the product of C-H functionalization and regenerates the Rh2(OAc)4 catalyst. The exceptional reactivity of Rh2 catalysts in these reactions has been ascribed to Rh-Rh interaction via the M-M bond. The Rh-Rh bond in the resulting intermediate acts as an electron reservoir; while one metal serves as a binding site for substrate, the second metal center shuttles electron density to and from the active metal center during substrate activation. The d-orbital splitting diagram of the intermediate complex (Rh-Rh core bound to the carbenoid) shows that the frontier d-orbitals are non-bonding with respect to the active Rh center (Figure 9a). The electron density in both the σ and π non-bonding MOs is centered on the nucleophilic carbenoid carbon and the "spectator" Rh center, which is not directly bound to the carbenoid unit (Figure 9b)4.

Figure 8. C-H bond functionalization via a metal-carbenoid intermediate.

Figure 9. (a) d-orbital MO splitting diagram of the Rh-Rh core in paddlewheel complexes bound to a carbenoid substrate. Note that only orbitals involved in substrate binding are shown. (b) The resulting σ and π non-bonding MOs are filled with electrons. The electron density in those MOs is centered on the carbenoid carbon and the "spectator" Rh center.
Paddlewheel complexes have also been utilized as building blocks in MOFs. MOFs are porous coordination polymers that consist of metal complexes linked together by organic ligands. The resulting one-, two-, or three-dimensional superstructures can be used in a variety of applications ranging from gas absorption (including separation and purification) to catalysis.
References
- Nguyen, T., Sutton, A. D., Brynda, M., Fettinger, J. C., Long, G. J., Power, P. P. Synthesis of a stable compound with fivefold bonding between two chromium(I) centers. Science. 310(5749), 844-847 (2005).
- Lin, C., Protasiewicz, J. D., Smith, E. T., Ren, T. Linear free energy relationship in dinuclear compounds. 2. Inductive redox tuning via remote substituents in quadruply bonded dimolybdenum compounds. Inorg Chem. 35(22), 6422-6428 (1996).
- Cotton, F. A., Murillo, C. A., Walton, R. A. Eds. Multiple Bonds Between Metal Atoms, 3rd ed. Springer. New York, NY. (2005).
- Nakamura, E., Yoshikai, N., Yamanaka, M. Mechanism of C−H Bond Activation/C−C Bond Formation Reaction between Diazo Compound and Alkane Catalyzed by Dirhodium Tetracarboxylate. J Am Chem Soc. 124 (24), 7181-7192 (2002).
Transcript
Paddlewheel complexes are a class of compounds comprised of two metal ions held in proximity to each other by four bridging ligands. Depending on their properties, paddlewheel complexes are used as catalysts or building blocks for metal-organic frameworks, also known as MOFs.
The M-M bonding in a paddlewheel complex affects the structure and reactivity of the compound, and can be further modified by variation of the metal ion and ligands.
In order to understand these properties, it is crucial to comprehend the electronic structure of the M-M bond in a given paddlewheel complex.
This video will illustrate the principles of M-M bonding, the synthesis and analysis of a dinuclear molybdenum complex, and various applications of paddlewheel complexes.
The M-M bond in a paddlewheel complex can be explained using molecular orbital theory.
When d-orbitals of two transition metals overlap, a M-M bond is formed. Depending on the orbital symmetry, three types of bonds can be created: σ, π, and δ bonds.
If the z axis is assigned to the M-M bond, both dz2 orbitals overlap head-on to form a σ bond. Overlap between two lobes of the dxz or dyz orbitals creates a π bond. Overlap between all four lobes of the dxy or dx2-y2 orbitals creates a δ bond.
The dx2-y2 orbital forms strong M-L bonds and usually does not contribute to M-M bonding. Hence, the maximum bond order achievable in many complexes is four.
Now, let’s take a look at the M-M bond in a dimolybdenum complex. First, assign the axes and highest available symmetry.
The z-axis describes the highest rotational symmetry, which is the C4 axis lying along the Mo-Mo bond. Next, assign the x- and y-axis, which lie along the Mo-N bonds.
As seen, the dx2-y2 orbital on each Mo atom is involved in M-L bonding, leaving the dxy, dxz, dyz, and dz2 orbitals for M-M bonding. This can be further described with an MO diagram.
Linear combination of the dz2 orbital on each metal atom results in σ and σ* molecular orbitals, while dxz and dyz orbitals form π and π* MOs. Finally, linear combination of dxy atomic orbitals creates the δ and δ* MOs. Filling the MOs with the d electrons of the Mo centers results in a quadruple bond.
M-M bonds can be measured using X-ray crystallography. To normalize for atomic radius, the formal shortness ratio is calculated with this equation. The FSR describes the ratio of the bond distance in the solid state to the sum of the atomic radii of the individual atoms, and is used to analyze and compare bonds in different metal complexes.
Now that you understand what quadruple bonds are and how to analyze them, let’s use this knowledge in a real example.
To begin, combine 6.0 g of p-anisidine and 4.2 mL of triethylorthoformate in a 100 mL round bottom flask with a magnetic stir bar. Attach a distillation head to the reaction flask, and place a beaker at the end of it.
Turn on the stirrer and hot plate. Collect the distilling byproduct ethanol in the beaker, and turn off the heat when ethanol distillation ceases.
Remove the flask from the oil bath and allow the reaction mixture to cool to room temperature. A precipitate should form. If the product does not precipitate, place the flask in an ice bath and scratch the bottom of the flask with a spatula to encourage crystallization.
Recrystallize the product from a minimal amount of boiling toluene. Collect the product by filtration through a fritted funnel and wash with 10 mL of hexanes.
Isolate the white product and allow it to dry in air in recrystallization dish. Lastly, using CDCl3, obtain a 1H NMR of the solid.
Before you start the synthesis, set up the Schlenk Line, ensuring N2 flow and a filled cold trap.
Familiarize yourself with the safety precautions using Mo(CO)6, which is highly toxic, and the Schlenk line techniques.
First, add 1.0 g of the freshly synthesized ligand and 0.34 g Mo(CO)6 to a 100 mL Schlenk flask and prepare the Schlenk flask for the cannula transfer of solvent.
Next, using cannula transfer add 20 mL of degassed o-dichlorobenzene to the Schlenk flask. Fit the Schlenk flask with a condenser connected to N2, and place the flask into a silicone-oil bath. Reflux the reaction for 2 h at 180 °C.
When finished, remove the Schlenk flask from the oil bath and allow the mixture to cool to room temperature. Once cooled, promptly filter the brown solution through a fritted funnel, to reduce the rate of product oxidation in presence of air.
Wash the yellow precipitate with 10 mL of hexanes, followed by 5 mL of reagent grade acetone. Collect the yellow, solid product and allow it to dry on air. Using CDCl3, measure the 1H NMR spectrum of the product.
First, degas the 20 mL of CH2Cl2 to minimize the rate of product oxidation by bubbling N2 through it for 10 minutes. Then, dissolve 20 mg of the product in 2 mL of degassed CH2Cl2 to make a saturated solution.
Next, insert a small piece of a low-lint wipe into a pipette to make a Celite plug. Add a small amount of Celite to the pipette. Filter the saturated solution of product in CH2Cl2 through the plug into a 5 mL vial. Use a pipette bulb to carefully push the solution through the plug.
Using tweezers, insert the 5 mL vial into a 10 mL scintillation vial. Add 2 mL of hexanes to the outer scintillation vial. Cap it tightly and place it on a shelf where the scintillation vial will not be disturbed.
Wait at least 24 hours to allow for single crystal growth, then collect single crystal X-ray data on the sample. Now that all the data is collected, let’s take a look at the results.
The ligand exhibits a characteristic peak for the NHC-HN bond at 8.02 ppm. The aromatic peaks integrate to 8H, and the two methoxy groups integrate to 6H total at 3.80 ppm.
In comparison, the singlet for the NHC-HN bond in the product occurs at 8.37 ppm and integrates to 4H. The doublets from the aromatic hydrogens are located at 6.49 and 6.16 ppm with a total integration of 32H. Lastly, the methoxy-groups are found at 3.70 ppm with an integration of 24H.
The two signals in the aromatic region indicate the 4-fold symmetry of the product. Additionally, the solid-state structure is consistent with the D4 point group and features a short Mo-Mo bond of 2.0925(3) Å.
Using the atomic radius of Mo, the FSR value for the M-M bond is calculated to be 0.72, which is consistent with the presence of a M-M quadruple bond.
Paddlewheel complexes, such as the dinuclear molybdenum complex synthesized in this video, display a wide range of properties and thus find application in diverse areas of chemistry.
For example, M-M bonds play an important role in catalysis. The paddlewheel complex Rh2(OAc)4 is a known catalyst for C-H bond functionalization via carbene and nitrene transfer reactions.
In a typical carbene transfer reaction, Rh2(OAc)4 reacts with a diazo compound to generate a Rh2 carbene intermediate. Subsequent insertion of the carbene into a C-H bond generates the product of C-H functionalization and regenerates the Rh2(OAc)4 catalyst.
Metal-organic frameworks, also known as MOFs, are porous compounds made of metal clusters linked together by organic ligands. This type of compound is a subclass of coordination polymers and can form one-, two-, or three-dimensional superstructures.
MOFs are used in many fields. Due to their high porosity and their large surface area per volume, MOFs find applications ranging from catalysts to gas storage and separation.
You’ve just watched JoVE’s introduction to quadruply M-M bonded complexes. You should now understand what quadruple M-M bonds are, how to synthesize paddlewheel complexes, and how to analyze them. Thanks for watching!
