18.8:
Batteries and Fuel Cells
18.8:
Batteries and Fuel Cells
A battery is a galvanic cell that is used as a source of electrical power for specific applications. Modern batteries exist in a multitude of forms to accommodate various applications, from tiny button batteries such as those that power wristwatches to the very large batteries used to supply backup energy to municipal power grids. Some batteries are designed for single-use applications and cannot be recharged (primary cells), while others are based on conveniently reversible cell reactions that allow recharging by an external power source (secondary cells).
Single-Use (Primary) Batteries
A dry-cell is a common primary battery that uses zinc as both the container and anode (“–” terminal) and a graphite rod as the cathode (“+” terminal). The Zn can is filled with an electrolyte paste containing manganese(IV) oxide, zinc(II) chloride, ammonium chloride, and water. A graphite rod is immersed in the electrolyte paste to complete the cell. The spontaneous cell reaction involves:
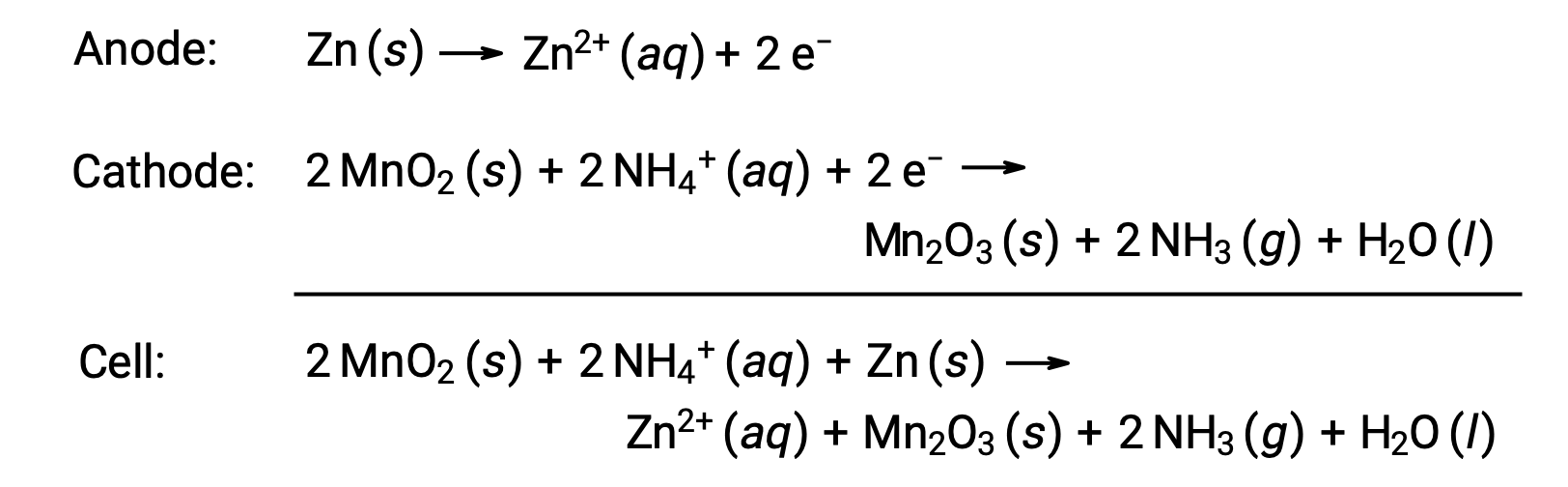
The voltage (cell potential) of a dry cell is approximately 1.5 V (Ecell ~ 1.5 V). Dry cells are available in various sizes (e.g., D, C, AA, AAA). All sizes of dry cells comprise the same components and exhibit the same voltage, but larger cells contain greater amounts of the redox reactants and therefore are capable of transferring correspondingly greater amounts of charge. Like other galvanic cells, dry cells may be connected in series to yield batteries with greater voltage outputs, if needed.
Alkaline batteries were designed around the same redox couples as the dry cell. As their name suggests, these types of batteries use alkaline electrolytes, often potassium hydroxide. The reactions are
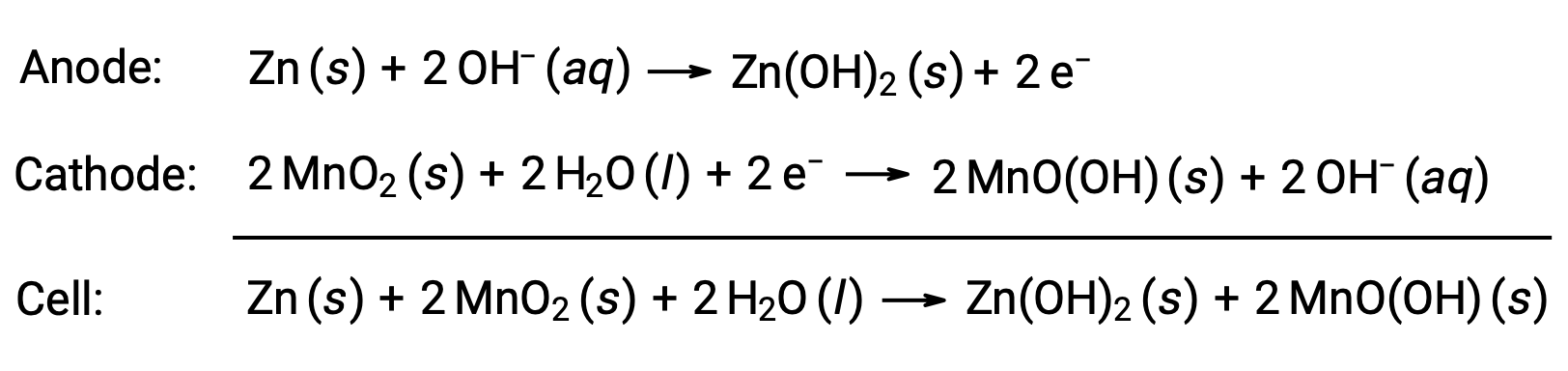
An alkaline battery can deliver about three to five times the energy of a dry cell of similar size (Ecell = +1.43 V). Alkaline batteries are prone to leaking potassium hydroxide, so they should be removed from devices for long-term storage. While some alkaline batteries are rechargeable, most are not.
Rechargeable (Secondary) Batteries
Nickel-cadmium, or NiCd, batteries consist of a nickel-plated cathode, cadmium-plated anode, and a potassium hydroxide electrode. The positive and negative plates, which are prevented from shorting by the separator, are rolled together and put into the case. This design allows the NiCd cell to deliver much more current than a similar-sized alkaline battery. The reactions are
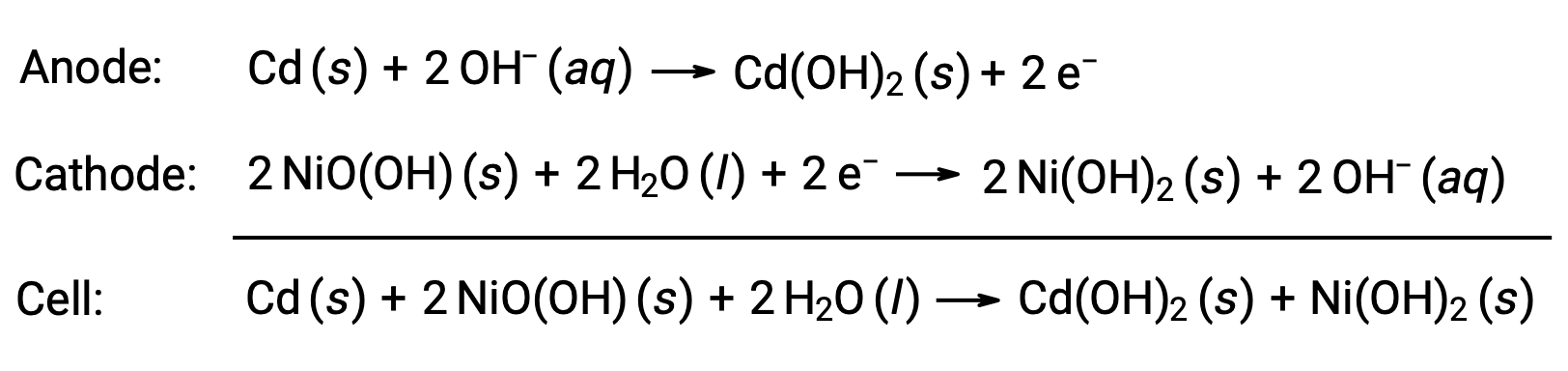
When properly treated, a NiCd battery can be recharged about 1000 times ( Ecell ~ 1.3 V). Cadmium is a toxic heavy metal, so NiCd batteries should never be ruptured or incinerated, and they should be disposed of in accordance with relevant toxic waste guidelines.
Lithium-ion batteries are among the most popular rechargeable batteries and are used in many portable electronic devices. The reactions are
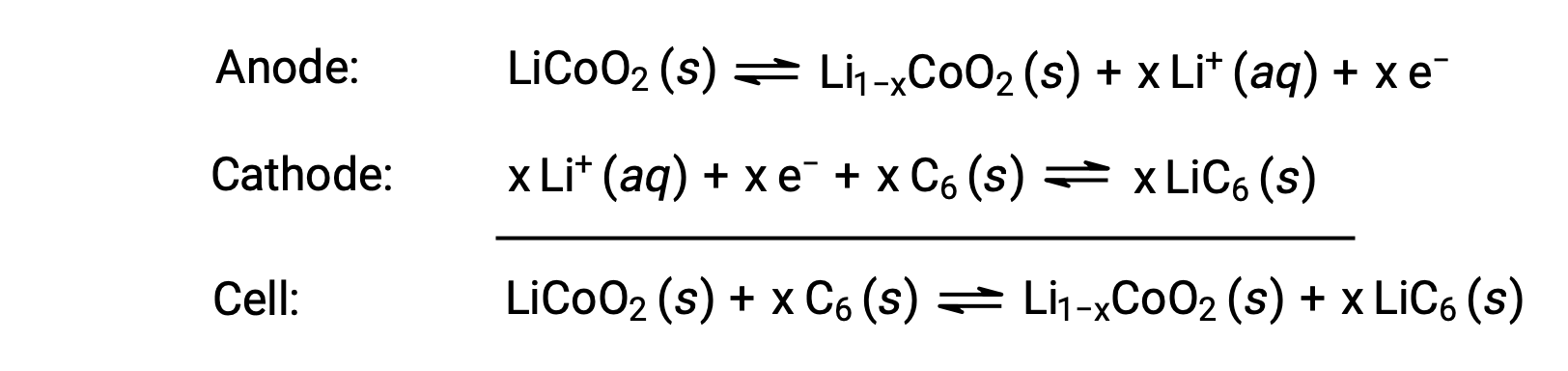
The variable stoichiometry of the cell reaction leads to variation in cell voltages, but for typical conditions, x is usually no more than 0.5,and the cell voltage is approximately 3.7 V (Ecell ~ 3.7). Lithium batteries are popular because they can provide a large amount of current, are lighter than comparable batteries of other types, produce a nearly constant voltage as they discharge, and only slowly lose their charge when stored.
The lead-acid battery is also a type of secondary battery commonly used in automobiles. It is inexpensive and capable of producing the high current required by automobile starter motors. The reactions for a lead-acid battery are
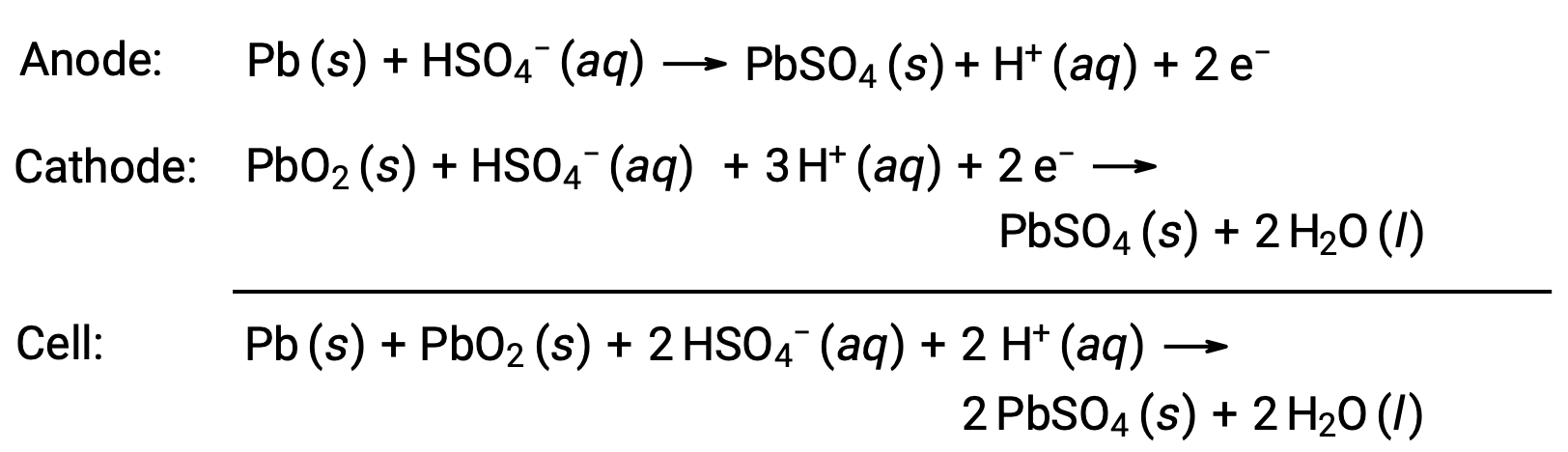
Each cell produces 2 V (Ecell ~ 2 V), so six cells are connected in series to produce a 12-V car battery. Lead-acid batteries are heavy and contain a caustic liquid electrolyte, H2SO4 (aq), but are often still the battery of choice because of their high current density. Since these batteries contain a significant amount of lead, they must always be disposed of properly.
Fuel Cells
A fuel cell is a galvanic cell that uses traditional combustive fuels, most often hydrogen or methane, that are continuously fed into the cell along with an oxidant. Within the cell, fuel and oxidant undergo the same redox chemistry as when they are combusted, but via a catalyzed electrochemical that is significantly more efficient. For example, a typical hydrogen fuel cell uses graphite electrodes embedded with platinum-based catalysts to accelerate the two half-cell reactions:
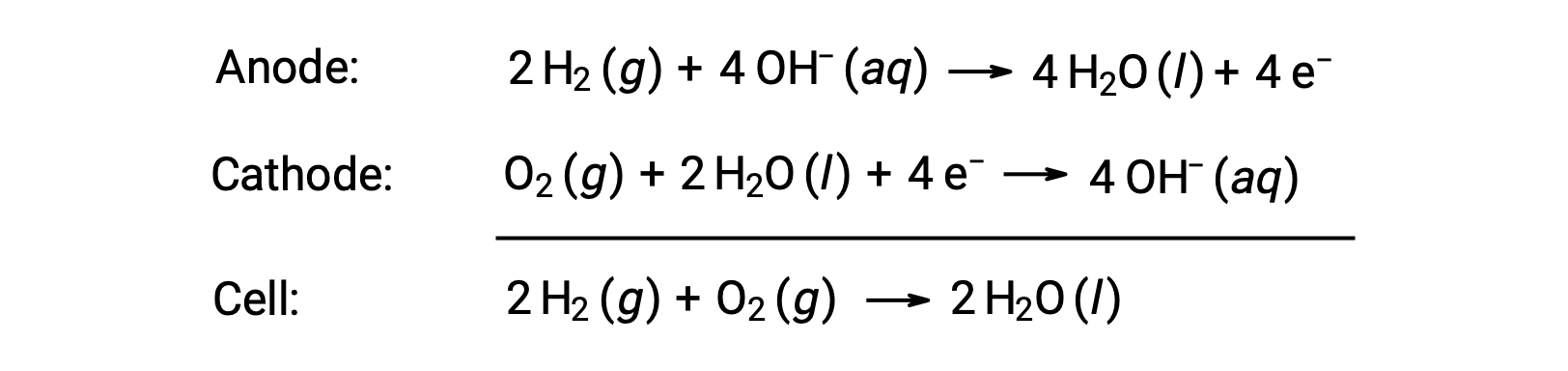
These types of fuel cells generally produce voltages of approximately 1.23 V (Ecell ~ 1.23 V). Compared to an internal combustion engine, the energy efficiency of a fuel cell using the same redox reaction is typically more than double (~20%–25% for an engine versus ~50%–75% for a fuel cell). Hydrogen fuel cells are commonly used on extended space missions, and prototypes for personal vehicles have been developed.
This text is adapted from Openstax, Chemistry 2e, Section 17.5: Batteries, and Fuel Cells.
