An Ex vivo Culture System to Study Thyroid Development
Summary
This protocol describes dissection of mouse embryonic thyroid anlagen and the culture of explants on semiporous filters or on microscopy plastic slides. This system is ideal to study morphogenetic or differentiation events occurring during thyroid development of wild type or knockout embryos, and is amenable to gain- and loss-of-function experiments.
Abstract
The thyroid is a bilobated endocrine gland localized at the base of the neck, producing the thyroid hormones T3, T4, and calcitonin. T3 and T4 are produced by differentiated thyrocytes, organized in closed spheres called follicles, while calcitonin is synthesized by C-cells, interspersed in between the follicles and a dense network of blood capillaries. Although adult thyroid architecture and functions have been extensively described and studied, the formation of the “angio-follicular” units, the distribution of C-cells in the parenchyma and the paracrine communications between epithelial and endothelial cells is far from being understood.
This method describes the sequential steps of mouse embryonic thyroid anlagen dissection and its culture on semiporous filters or on microscopy plastic slides. Within a period of four days, this culture system faithfully recapitulates in vivo thyroid development. Indeed, (i) bilobation of the organ occurs (for e12.5 explants), (ii) thyrocytes precursors organize into follicles and polarize, (iii) thyrocytes and C-cells differentiate, and (iv) endothelial cells present in the microdissected tissue proliferate, migrate into the thyroid lobes, and closely associate with the epithelial cells, as they do in vivo.
Thyroid tissues can be obtained from wild type, knockout or fluorescent transgenic embryos. Moreover, explants culture can be manipulated by addition of inhibitors, blocking antibodies, growth factors, or even cells or conditioned medium. Ex vivo development can be analyzed in real-time, or at any time of the culture by immunostaining and RT-qPCR.
In conclusion, thyroid explant culture combined with downstream whole-mount or on sections imaging and gene expression profiling provides a powerful system for manipulating and studying morphogenetic and differentiation events of thyroid organogenesis.
Introduction
The thyroid gland is a collection of independent epithelial spheres, called follicles, surrounded by a dense network of endothelial capillaries. This organization allows thyroid function: endothelial capillaries provide thyrocytes with iodine, required for T3 and T4 hormone synthesis, and distribute these latter to the whole body. Scattered in between the follicles and capillaries, C-cells produce the hypocalcemic hormone calcitonin1. Although adult thyroid architecture and functions are well known, the cellular and molecular mechanisms involved in thyroid embryonic development (follicle formation and differentiation) are far from being understood.
During embryogenesis, thyrocytes progenitor originates as a thickening (the midline anlage) of the ventral wall of the foregut endoderm at embryonic day (e) 8.5 in the mouse embryo, while C-cells progenitors originate at e11.5 as droplet-shaped protrusions (the ultimobranchial bodies) of the fourth pharyngeal pouches2-6. The midline bud then detaches from the endoderm, expands bilaterally to fuse at e13.5 with the ultimobranchial bodies on each side of the trachea. Finally, thyrocytes organize into follicles and C-cells differentiate.
Current knowledge on thyroid formation mainly comes from histological analysis of fixed tissues, but the morphogenetic events involved in thyroid formation are highly dynamic and involve communications and interactions between different cell types and with the extracellular matrix. Recent work showed that thyrocyte progenitors produce high levels of VEGF to recruit endothelial cells to the developing thyroid, and, in turn, recruited endothelial cells promote follicle formation and C-cells differentiation7.
Most ex vivo studies on the thyroid have been performed on isolated adult thyroid-derived cells, grown either on 2D tissue culture plastic dishes or in 3D matrices. Using these types of cultures, differentiated follicular cells either remain polarized and organized as follicles or reacquire a 3D organization8-10. However, these pure epithelial cells, explanted from their physiological environment, and cultured in 2D, ignore interactions with extracellular matrix, cytokines, growth factors and with other cell types such as the endothelial or nerves cells that they normally encounter in vivo. A very nice study recently described a differentiation protocol of ES cells into thyroid follicles using a final culture step in 3D matrigel11. However, these 3D cultures lack contact with other cell types.
Based on previous expertise on pancreas and salivary glands organ culture12-14, a method for dissecting mouse embryonic thyroid anlagen and culturing the explants on semiporous filters or on microscopy plastic slides was developed.
When working with e12.5 embryos, the dual origin of the thyroid anlagen (the midline anlage and the two lateral ultimobranchial bodies) imposed the microdissection of a large fragment of tissue. This contained the trachea, but not the esophagus, and extended from the pharyngeal arch arteries up to the arytenoid swelling. When cultured on filters, the midline anlage extends laterally on each side of the trachea, where they fuse with the ultimobranchial bodies to form the two thyroid lobes, still connected by a narrow isthmus.
In culture, epithelial cells proliferate, organize into follicles and differentiate into thyrocytes and C-cells, depending on their origin. Endothelial cells contained in the microdissected tissue also proliferate and invade the thyroid lobes to finally associate closely with the epithelial follicular structure, independently of blood flow or circulating factors. As development of the explants faithfully recapitulates in vivo development, this culture system is optimal to study morphogenetic and differentiation events occurring during thyroid development.
Thyroid tissues can be obtained from wild type, knockout or fluorescent transgenic embryos, and the culture system is amenable to loss- and gain-of-function experiments. Finally, time-lapse imaging of fluorescently labeled thyroid explants on microscopy plastic dishes could be exploited to better investigate the kinetics and continuity of morphogenetic movements that occur in vivo. Time-lapse imaging has already been used to study branching morphogenesis of the pancreas15,16 or the ureteric bud17.
Protocol
Mice were raised and treated according to the principles of laboratory animal care of the University Animal Welfare Committee. All procedures and protocols were approved by this Committee.
1. Coating of Microscopy Plastic Culture Chambers
NOTE: Perform all the following steps under sterile conditions in a laminar flow hood. The two types of coating are functionally equivalent.
- Coat plastic culture chambers with fibronectin one day prior to isolation of thyroid tissues:
- Dilute sterile fibronectin to a 50 μg/ml final concentration in M199 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone and 2 mM glutamine12.
- Cover the wells with 200 μl of diluted fibronectin and incubate overnight in a 4 °C refrigerator.
- On the day of the dissection, aspirate the fibronectin, rinse the wells once with M199 and add a minimum volume of 200 μl of M199 supplemented with antibiotics, glutamine and 10% fetal calf serum.
- Leave the coated chambers in the incubator until ready to plate the explants.
- Coat plastic culture chambers with type I collagen 2 hr before isolation of thyroid tissues:
- Dilute sterile type I collagen to a 50 μg/ml final concentration in 10 mM HCl. NOTE: Keep collagen stock solution on ice.
- Cover the wells with 200 μl of diluted type I collagen and incubate for 2 hr at room temperature (RT).
- Aspirate the type I collagen solution, rinse the wells once with M199 and add a minimum volume of 200 μl of M199 supplemented with antibiotics, glutamine and 10% fetal calf serum.
- Leave the coated chambers in the incubator until ready to plate the explants.
2. Dissection of Thyroid Anlagen-containing Tissues (e12.5) or Thyroid Lobes (e13.5-e14.5)
NOTE: Use clean dissecting tools and Petri/culture plates. The general steps and incisions described below are the same for e12.5 and e14.5 embryos.
- Sacrifice timed-pregnant female mice at the desired embryonic (e) day (from e12.5 to e14.5) and remove the uterus using dissecting instruments.
- Place the dissected uterus in a 10 cm plate containing HBSS.
- Hold one extremity of the uterus with forceps and open the uterus with small scissors to liberate progressively all the embryos.
- Separate the embryos from the placenta with scissors and transfer the embryos with their extraembryonic membranes in a new 10 cm plate containing HBSS.
- Remove the yolk sac and the amnion. NOTE: From this point, work under a stereomicroscope with transillumination from below; increasing magnification with dissection steps. Use tungsten needles in glass capillaries as dissecting tools.
- Using the needles like a knife and fork, remove the upper part of the head, including the upper jaw and the ears (Figure 1A, cut along a).
- Hold the embryo on its back and remove first an extra piece of the neural tube (Figure 1A, cut along b), then the lower part of the embryo, just under the anterior limbs and above the heart (Figure 1B).
- Transfer the embryo slice containing the tongue (to orient the tissue) and neck region in a new 10 cm plate containing HBSS.
- Remove the neural tube and tissues posterior to the esophagus/trachea (Figure 1C, cut along a).
- Gently section the tissue slice along both sides of the tongue (Figure 1C, cut along b and b’).
- Locate the esophagus and trachea, in the prolongation of the tongue, and dissect away the tongue, leaving the arytenoid swelling (Figure 1D, cut along a), and the tissue on both sides of the esophagus/trachea (Figure 1D, cut along b and b’).
- Remove the esophagus, and place the trachea with its ventral side facing up.
- Dissect away the undesired tissues such as the thymus (Figure 1E) and all the tissues located laterally to the pharyngeal arch arteries. For e13.5 or e14.5 embryos, visualize the thyroid lobes on each side of the trachea (Figure 1F; red dotted lines) and further dissect the lobes from the trachea.
- Transfer the isolated e12.5 trachea region, or the e13.5-e14.5 thyroid lobes into a 35 mm culture plate containing pre-warmed M199 medium supplemented with antibiotics, glutamine and 10% fetal calf serum. Transfer explants using a prewetted filter tip on a P-200 micropipette. For e12.5 explants, cut the extremity of the tip to widen the opening. NOTE: Routinely, and if embryos have the same genotype, perform steps 6 & 7 on all the embryos, then 9-11, and finally 12 & 13.
3. Plating and Culture of Thyroid Explants
- Wash the explants by successive transfer in three wells of a 24-well plate containing 1 ml of pre-warmed culture medium (= M199 supplemented with antibiotics, glutamine and 10% fetal calf serum).
- Plating of thyroid lobes on coated microscopy plastic culture chambers:
- Aspirate M199 culture medium from the coated chambers.
- Carefully, transfer the thyroid explants into the culture chambers using a micropipette and filter tips. Place one explant in the center of each chamber.
- Remove excess medium and place the culture chamber in a tissue culture incubator (37 °C; 5% CO2) for 2 hr to let the explants attach to the matrix.
- Gently add 200 to 300 μl of pre-warmed culture medium to each well.
- Incubate for long-term culture in the tissue culture incubator.
- Plating of trachea region or thyroid lobes on semiporous filters:
- Fill the number of wells of a 24-well plate required with 330 μl of pre-warmed culture medium.
- Place filters (e.g. 0.4 µm Millipore filter) on the medium, taking care to avoid trapping of air bubbles below the culture filter.
- Carefully, using a micropipette and filter tips, transfer the thyroid explants with a minimal volume of medium onto the center of the filters (maximum 4/filter).
- Remove the excess of medium and space out the explants with a tungsten needle.
- Incubate for long-term culture in the tissue culture incubator. NOTE: Change culture medium every other day under the laminar flow hood. Culture the explants up to 7 days.
4. Whole-mount Immunofluorescence Staining Protocol for Thyroid Explants
Dislodge explants grown on filters (after step 2 below) using tungsten needles or fine forceps and transfer in a 24-well plate for all the steps, except for primary (step 5) and secondary (step 7) antibody incubations (96-well plate). For adherent explants cultured on microscopy plastic chambers, perform all the steps in the culture chambers.
- Aspirate M199 culture medium and fix the explant with ice-cold 4% paraformaldehyde (PFA) for 20 min (500 µl in 24-well dish and 300 µl in microscopy slides).
- Wash 2x 10 min in Tris Buffered Saline with Triton (TBST; 50 mM Tris HCl pH 7.5, 150 mM NaCl, 0,1% Triton X-100) at RT.
- Process the explants immediately or store at -20 °C. NOTE: For long-term storage at -20 °C, dehydrate the explants in methanol by gradually increasing methanol concentration in TBST to reach 100% methanol. When ready to continue with the immunostaining (step 4), rehydrate the explants by gradually adding TBST to methanol.
- Replace TBST with the blocking solution (10% normal Goat Serum in TBST) and incubate on a sea-saw rocker for 30-60 min at RT.
- Dilute primary antibody in blocking solution (100-200 µl per condition). Transfer the “filter” explants from a 24-well plate to a 96-well plate. Incubate explants with the primary antibodies on a sea-saw rocker overnight at 4 °C.
- The next day, discard the primary antibody solution, transfer the “filter” explants back in a 24-well plate, and wash at least five times with TBST on a sea-saw rocker for 45-60 min each at RT.
- Dilute secondary antibody (e.g. Goat anti-X-Alexa Fluor at 1:2,000 dilution) in TBST containing 1% of normal goat serum and incubate the explants with this dilution overnight on a sea-saw rocker at 4 °C and in the dark. For nuclear counterstaining, include 1 μg/ml of bis-Benzimide (Hoechst) together with the secondary antibody solution.
- The next day, discard the secondary antibody solution and wash 5x with TBST on a sea-saw rocker for 45-60 min each at RT. During the washes, keep the explants in the dark.
- Perform an additional wash in TBST gently swirling overnight at 4 °C in the dark.
- Postfix the immunostained explants in 4% PFA at 4 °C for 10 min.
- Wash 2x 10 min with TBST.
- Transfer the explants either gradually in methanol 100% for long-term storage at -20 °C, or analyze immediately. For explants cultured on filters, and immunostained in a 24-well plate, transfer in a microscopy chamber (e.g. LabTek or Ibidi) with TBST or better fluorescent mounting medium prior to analysis with a Multiphoton or confocal microscope.
5. Extraction of RNA from Thyroid
Work in an RNase-free environment. Therefore, use nucleic acid and nuclease free pipette tips and clean both bench and equipment from contaminants and RNases. This protocol being based on phenol/chloroform, perform the first steps (1 to 7) under a chemical hood.
- Homogenize one thyroid explant or two thyroid lobes with 300 µl of RNA extraction solution using a disposable pestle for 1.5 ml tube. In between samples, wash the pestle in 2% SDS, then water, then ethanol.
- Add another 100 µl of RNA extraction solution, vortex 10 sec and incubate at RT for 10 min.
- Vortex for 30 sec and add 20 ng of tRNA to each sample.
- Vortex for 1 min and add 80 µl of chloroform.
- Vortex vigorously for 30 sec and incubate 15 min at RT.
- Spin for 15 min at 19,000 x g in a refrigerated (4 °C) centrifuge.
- Transfer carefully the colorless upper aqueous phase in a fresh tube containing 20 μg of glycogen as coprecipitant.
- Vortex and add 200 µl of 100% isopropanol alcohol.
- Vortex vigorously for 15-30 sec and let RNA precipitate at -20 °C for 2-3 hr or at -80 °C for 1 hr.
- Spin for 30 min at 19,000 x g at 4 °C, and eliminate supernatant.
- Wash the pellet with 450 µl of cold 75% ethanol and vortex to dislodge the pellet.
- Spin for 10 min at 19,000 x g at 4 °C, and eliminate supernatant.
- Dry the pellet under the hood for 5 min.
- Resuspend the pellet with 10 µl of RNase-free water and incubate 10 min at RT.
- Assay RNA concentration, store at -80 °C or reverse transcribe for gene expression analysis.
Representative Results
Thyroid anlages (midline and UB, together with surrounding tissues), and thyroid lobes are dissected from mouse embryos at e12.5 and e13.5/e14.5, respectively (Figure 1). After one day in culture on filter, the midline anlage is visible (Figure 2A) as an elongated tissue spanning on top of the trachea. It progressively forms two lobes on each side of the trachea. If isolated thyroid lobes (e13.5 or e14.5) are cultured, they will expand, undergo morphogenesis and epithelial cells will organize into macroscopically visible follicular structures (Figure 2B).
Organization and polarization of epithelial cells, labeled with E-cadherin, can be visualized by ezrin immunolabeling (Figure 3A). Ezrin-positive intracellular structures progressively, and in a concerted way, fuse with one pole of the cell that will become the apical pole. During this process, endothelial cells positive for PECAM proliferate and organize around the developing follicles to form angio-follicular units (Figure 3B). To quantify differentiation of thyrocytes and C-cells, RNA can be isolated from the cultured thyroid lobes, and gene expression assayed by RT-qPCR (Figure 3C). While the expression of the thyroid transcription factor Nkx2.1 does not change during the culture, expression of thyrocyte-specific thyroglobulin and of C-cell-specific calcitonin dramatically increased, indicating functional differentiation.
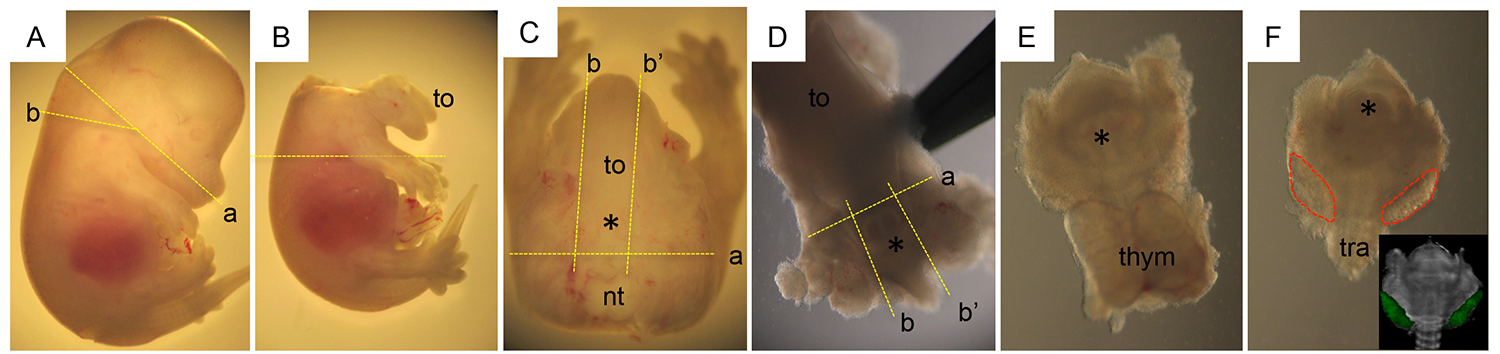
Figure 1. Dissection steps to isolate the thyroid from e14.5 mouse embryo. Incisions are depicted by yellow dotted lines. (A) The upper part of the head is removed by two incisions to expose the tongue. (B) The neck region is separated from the rest of the body by sectioning the embryo below the upper limbs and above the heart. (C) Keeping the tongue (to) as a guide, remove the neural tube (nt) and the lateral tissues and limbs. (D) The tongue, arytenoid swelling (*) and the esophagus/trachea are perfectly aligned. First remove the tongue and then tissues lateral to the esophagus/trachea region. (E) The thyroid lobes are partly hidden by the thymus (thym) which should be discarded. (F) Thyroid lobes are visible on each side of the trachea (red dotted ovoids), or better visualized using fluorescent reporter embryos (Pax8-Cre; Rosa-stop-YFP; inset). Abbreviations: to (tongue), thym (thymus), tra (trachea), * (arytenoid swelling). Please click here to view a larger version of this figure.
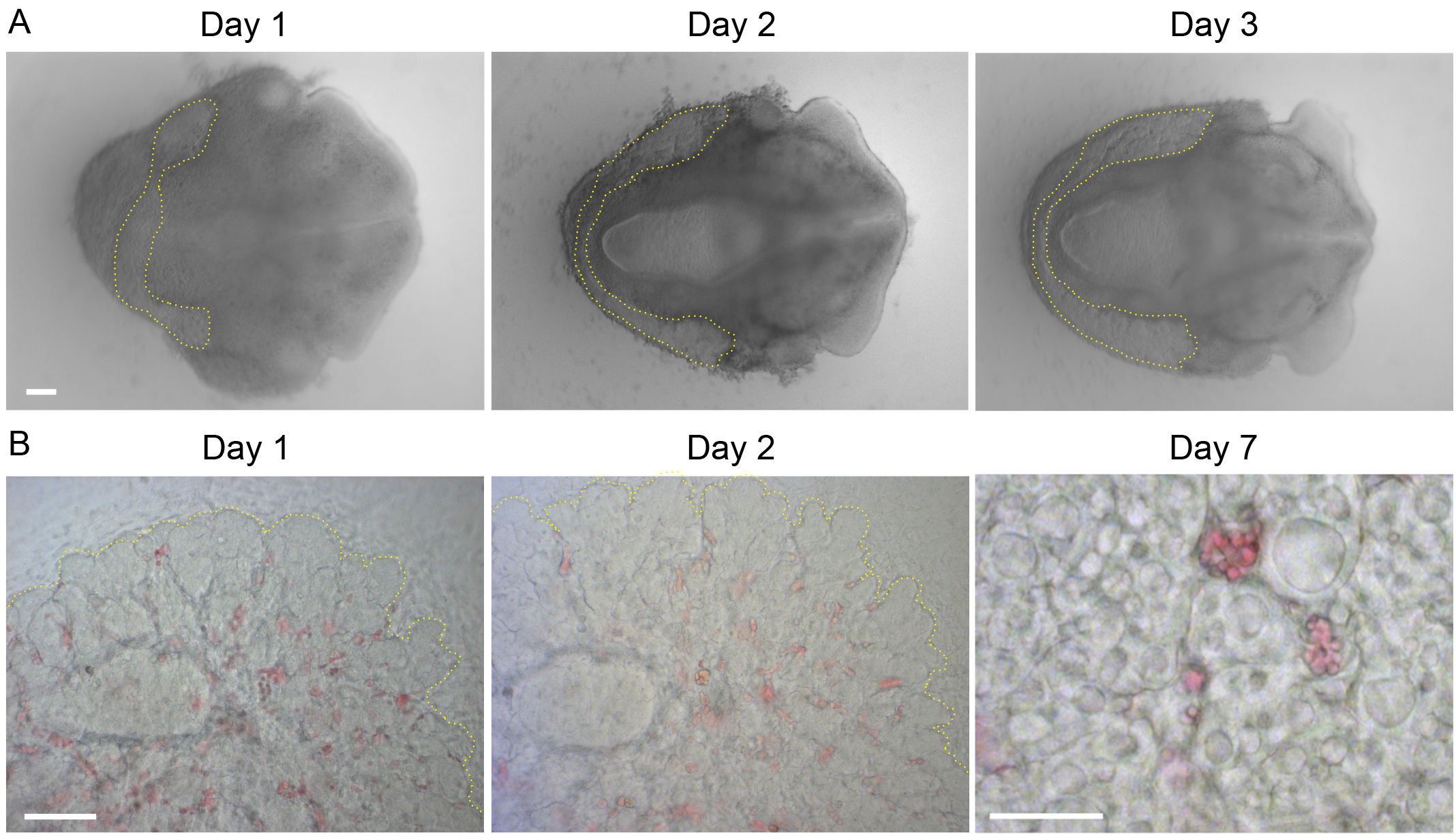
Figure 2. Dissected thyroid explants on filter and thyroid lobes on fibronectin nicely develop ex vivo. (A) Photographs of an e12.5 thyroid explant cultured for 1, 2 and 3 days on semiporous filter, at the air-medium interface. Thyroid epithelial cells migrate bilaterally on each side of the trachea and form two growing thyroid lobes. (B) Brightfield images on day 1 and day 2 of an e14.5 thyroid lobe cultured on microscopy plastic culture chambers. Note the expansion and remodeling of the epithelium that ultimately form large (20-100 μm) follicles around day 7. Yellow dotted line delineates the epithelial periphery of the thyroid. Scale bars, 100 μm. Please click here to view a larger version of this figure.

Figure 3. Folliculogenesis, angiogenesis and differentiation of thyroid in culture. Thyroid lobes were cultured for the indicated time. (A) Fixed tissues were whole-mount immunostained with antibodies against the basolateral marker E-cadherin, and the subapical membrane marker Ezrin. After one day in culture, epithelial cells of the thyroid parenchyma present small ezrin-positive structures that correspond to intracellular vesicles7. Concerted fusion of the vesicles from adjacent cells will form small lumen at day 2; their progressive growth, and organization of the surrounding cells around the lumen will delineate pre-follicular structures after 4 days. Scale bar, 20 μm. (B) Thyroid lobe immunostained with antibodies against the Platelet and Endothelial Cell Adhesion Molecule (PECAM) and Ezrin. Follicles are surrounded by endothelial structures. Scale bar, 50 μm. (C) RNA, extracted from thyroid lobes, was reverse transcribed before quantitative PCR. The expression of the thyroid transcription factor Nkx2.1, and of two differentiation genes, thyroglobulin and Calcitonin, was measured using specific primer pairs. To calculate the relative gene expression, the ΔΔ Ct method was used, using E-cadherin as the reference gene and the last time point of the culture as 100%. Images shown in A and B are single confocal optical sections of whole-mount immunostained thyroid lobes. Please click here to view a larger version of this figure.
Discussion
This paper describes a method for dissecting and culturing thyroid explants (e12.5) or lobes (e14.5) in order to study and better understand the complex events leading to thyroid formation. Due to the dual origin of the thyroid anlagen (the midline anlage and the two lateral ultimobranchial bodies), and their small size, a fragment of e12.5 tissue localized rostral to the pharyngeal arch arteries, and containing the trachea, but not the esophagus is microdissected. As illustrated, within a period of four days, this culture system faithfully recapitulates in vivo thyroid development since: (i) the midline anlage migrates bilaterally and expands to form, with the UB, two thyroid lobes connected by a narrow isthmus (Figure 2A), (ii) thyroid precursors reorganize from a mass of cells into follicles and ultimately polarize (Figures 2B and 3A), (iii) endothelial cells present in the microdissected tissue proliferate, migrate into the thyroid lobes, and closely associate with the epithelial cells (Figure 3B), and (iv) thyrocytes and C-cells differentiate qualitatively (Figure 3C), as they do in vivo7. As dissection physically interrupts in utero development and explanted tissue has to adapt to culture condition, the morphological and differentiation events are slightly delayed as compared to the in vivo situation.
Critical steps within the protocol
The critical aspect of this method is in the dissection. First, it is essential to remove lateral tissues, as well as the right and left lobes of the thymus gland from the e12.5 explants, as their expansion in culture hinder proper development of the thyroid lobes. Second, rapidity is also critical if one wants to maintain endothelial cells alive. If dissection is not performed under a sterile atmosphere, it is important to wash several times the explants in sterile culture medium. Finally, when culturing explants on semiporous filters, it is important to use a small volume of medium and to place the explants on the center of the filter. Too much medium will flow to the periphery of the filter and create a meniscus with the filter wall. In this case, the explant will follow the medium and reaches the meniscus; its development will be in medium rather than at the air/medium interface.
Possible modifications and limitations
The protocol described here applies to e12.5 thyroid explants and to e13.5 and e14.5 isolated thyroid lobes. Younger and older developmental stages could be tested in function of the scientific question addressed. Other culture conditions could also be tested: thyroid explants or lobes could be grown in 3D matrigel, or the medium could be complemented with Insulin/Transferrin/Selenium rather than with 10% serum.
An important aspect of tissue culture is oxygen concentration. When grown on microscopy plastic slides, oxygen concentration is difficult to appreciate as it will decrease with the height of medium above the explants. When grown on filters, at the air/medium interface, explants are in contact with the atmosphere, hence with 21% of oxygen. In this latter case, one could try to reproduce the “hypoxic” in utero environment using lower pO2 concentration inside the incubator.
If tissular complexity of the e12.5 explants is an advantage for correct development, it is also a main drawback as the epithelial cells are not accessible to viruses or transfection reagents. Manipulation can be performed with diffusible reagents (inhibitors, blocking antibodies, growth factors, cytokines, conditioned medium), keeping in mind that the exogenous compound affects all cell types. Moreover, tissue complexity and the small size of the lobes relative to the entire tissue explant (see Figure 2A, right panel), reduces the purity of the extracts (RNA or protein) prepared.
Although most of the data presented in this paper were obtained from 4-5 days cultured explants, it is possible to keep the organ in culture for longer time, up to 10 days on plastic, with a continuous development of the follicles (see Figure 2B at day 7). If longer cultures have to be performed, one should verify that all the cell types, including endothelial cells, survive in the explants.
Significance of the technique with respect to existing methods or other alternative methods
Compared to culture of pure populations of thyrocytes in 2D dishes or 3D matrices, this organ culture system presents the advantage of maintaining the natural and physiological composition of the microdissected fragments. Indeed, in addition to thyrocytes and C-cells progenitors, explants contain mesenchymal and endothelial cells as well as extracellular matrix. As already demonstrated with salivary glands, kidney, lung or pancreas, organ cultures nicely mimic in vivo development. Moreover, it provides a way to rapidly analyze and manipulate (gain or loss-of-function) wild type and knockout organs.
Future applications or directions after mastering this technique
This paper shows that the cellular and molecular aspects of thyroid development can be analyzed with a microscope or by RT-qPCR; in situ hybridization or western blotting could also be performed. This culture system is amenable to gain- and loss-of-function experiments via the addition of specific inhibitors, blocking antibodies, growth factors, cytokines, or even cells in the culture medium7. However, all the cell types present in the tissue are affected. It would be interesting to develop virus-based cell-type specific targeting.
The use of a fluorescent reporter strain, with a fluorescent dissecting microscope, facilitates the microdissection of the tissue piece (Figure 1F, inset), but also, as reported by others using a membrane-tagged fluorescent protein16, allows to perform real-time imaging of the developing organ in 3D15,16, or to follow a particular cell population in the culture. Development of new fluorescent reporter strains will be necessary to carefully visualize morphogenetic events.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The work was supported by grants from the Université catholique de Louvain (Action de recherché concertées) and the Fund for Scientific Medical Research (F.R.S.-FNRS, Belgium). A.-S.D. is a doctoral fellow from Télévie, A.-C.H. held a fellowship from the Fonds pour la formation à la Recherche dans l’Industrie et dans l’Agriculture (Belgium), M.V. is supported by the de Duve Institute and C.E.P. is a senior research associate of the F.R.S.-FNRS (Belgium).
Materials
| µ-slide 8 well, ibiTreat, tissue culture treated, sterile | proxylab | 80826 | |
| Collagen Type I, rat tail | Millipore | 08-115 | |
| Fibronectin from human plasma | Invitrogen | # 33010-018 | |
| M199 | Invitrogen | 31150-022 | |
| HBSS | Invitrogen | 14025-100 | |
| Tungsten wire | Goodfellow | LS237450 | |
| culture plate insert (12 mm diameter) | Millipore | PICM01250 | |
| GlycoBlue | Invitrogen | AM9516 | |
| E-cadherin | BD Biosciences | 610182 | 1/1000 |
| Ezrin | Thermo Scientific | MS-661-P1 | 1/400 |
| PECAM | BD Biosciences | 550274 | 1/100 |
| Hoechst | Sigma | B2261 |
References
- Colin, I. M., Denef, J. -. F., Lengelé, B., Many, M. -. C., Gérard, A. -. C. Recent insights into the cell biology of thyroid angiofollicular units. Endocr. Rev. 34 (2), 209-238 (2013).
- Fagman, H., Andersson, L., Nilsson, M. The developing mouse thyroid embryonic vessel contacts and parenchymal growth pattern during specification budding, migration, and lobulation. Dev. Dyn. 235 (2), 444-455 (2006).
- Fagman, H., Nilsson, M. Morphogenesis of the thyroid gland. Mol. Cell. Endocrinol. 323 (1), 35-54 (2010).
- Fagman, H., Nilsson, M. Morphogenetics of early thyroid development. J. Mol. Endocrinol. 46 (1), (2011).
- De Felice, M., Di Lauro, R. Thyroid development and its disorders genetics and molecular mechanisms. Endocr. Rev. 25 (5), 722-746 (2004).
- De Felice, M., Di Lauro, R. Intrinsic and extrinsic factors in thyroid gland development: an update. Endocrinology. 152 (8), 2948-2956 (2011).
- Hick, A. -. C., et al. Reciprocal epithelial paracrine interactions during thyroid development govern follicular organization and C-cells differentiation. Dev. Biol. 381 (1), 227-240 (2013).
- Toda, S., Koike, N., Sugihara, H. Cellular integration of thyrocytes and thyroid folliculogenesis: a perspective for thyroid tissue regeneration and engineering. Endocr. J. 48, 407-425 (2001).
- Toda, S., et al. Culture models for studying thyroid biology and disorders. ISRN Endocrinol. , (2011).
- Eggo, M. C., Quiney, V. M., Campbell, S. Local factors regulating growth and function of human thyroid cells in vitro and in vivo. 213, 47-58 (2003).
- Antonica, F., et al. Generation of functional thyroid from embryonic stem cells. Nature. 491, 66-71 (2012).
- van Eyll, J. M., Pierreux, C. E., Lemaigre, F. P., Rousseau, G. G. Shh-dependent differentiation of intestinal tissue from embryonic pancreas by activin A. J. Cell Sci. 117, 2077-2086 (2004).
- Hick, A. -. C., et al. Mechanism of primitive duct formation in the pancreas and submandibular glands: a role for SDF-1. BMC Dev. Biol. 9. , (2009).
- Pierreux, C. E., et al. Epithelial:Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev. Biol. 347, 216-227 (2010).
- Puri, S., Hebrok, M. Dynamics of embryonic pancreas development using real-time imaging. Dev. Biol. 306, 82-93 (2007).
- Petzold, K. M., Spagnoli, F. M. A system for ex-vivo culturing of embryonic pancreas. J. Vis. Exp. , (2012).
- Watanabe, T., Costantini, F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev. Biol. 271, 98-108 (2004).

