A Simple Behavioral Assay for Testing Visual Function in Xenopus laevis
Summary
Xenopus laevis tadpoles prefer swimming on the white side of a black/white tank. This behavior is guided by their vision. Based on this behavior, we present a simple assay to test the visual function of tadpoles.
Abstract
Measurement of the visual function in the tadpoles of the frog, Xenopus laevis, allows screening for blindness in live animals. The optokinetic response is a vision-based, reflexive behavior that has been observed in all vertebrates tested. Tadpole eyes are small so the tail flip response was used as alternative measure, which requires a trained technician to record the subtle response. We developed an alternative behavior assay based on the fact that tadpoles prefer to swim on the white side of a tank when placed in a tank with both black and white sides. The assay presented here is an inexpensive, simple alternative that creates a response that is easily measured. The setup consists of a tripod, webcam and nested testing tanks, readily available in most Xenopus laboratories. This article includes a movie showing the behavior of tadpoles, before and after severing the optic nerve. In order to test the function of one eye, we also include representative results of a tadpole in which each eye underwent retinal axotomy on consecutive days. Future studies could develop an automated version of this assay for testing the vision of many tadpoles at once.
Introduction
Xenopus laevis have been used as a model organism to study eye formation. The eyes develop quickly, growing to maturity in less than a week for testing genes or pathways that have an effect on visual development and function. To test visual function, the optokinetic and optomotor response has been used in zebrafish and Xenopus tadpoles, respectively1,2 . Because the eyes of Xenopus tadpoles are relatively smaller than zebrafish, this assay requires the use of specialized equipment and trained personnel to detect the subtle tail-flip and eye-movement behavior in Xenopus. A more robust behavior in Xenopus is the preference for swimming in a tank with a white background, which is described herein3. When placing a tadpole in a half black/half-white tank, the pre-metamorphic tadpole quickly swims to the white side of the tank. We previously used this assay to determine if pluripotent cell-derived eyes were functional4. Here, we report a detailed version of this assay, which can be used to test the visual function of premetamorphic Xenopus tadpoles.
This assay is simpler than the optomotor response assay, since it only requires a mounted digital camcorder, digital camera software and standard equipment found in most Xenopus laboratories. In addition, the recorded response requires no special training to tally results. Our representative results show that the same group of tadpoles, having undergone double retinal axotomy, swim randomly around the tank. We have also included the behavior assay results from a representative tadpole, showing how one eye can be tested for visual response. A worksheet has been included so that numbers acquired during the assay can be inserted and analyzed. This worksheet can be used to determine whether the tadpoles tested have a visual response.
Protocol
Animal Care
The Xenopus laevis tadpoles used in this study were grown, raised and treated according to procedures approved by Upstate Medical University IACUC and the Guide for the Care and Use of Laboratory Animals.
1. Pre-behavior Setup: Tadpoles
- Obtain fertilized Xenopus embryos from a commercially available source or in vitro fertilize oocytes, as previously described6.
- Place blastula to neural plate stage embryos at 18 ˚C in 60 mm Petri dishes containing 0.1X MMR [10 X Marc's Modified Ringer's (MMR) solution stock (10 mM MgCl2; 20 mM KCl; 20 mM CaCl2 ; 50 mM HEPES; 1 M NaCl; adjusted to pH 7.5, autoclaved and stored at RT)] with 50 µg/ml gentamicin antibiotic. Change 0.1X MMR each day and remove any dead embryos.
- Once the embryos reach Nieuwkoop and Faber stage ~27 – 307, switch the embryos into 0.1X MMR without antibiotic to allow gut bacterial growth.
- Grow the tadpoles until they reach the feeding stage (stage 45) and move them into 100 mm Petri dishes filled with 0.1X MMR. Feed them nettle powder supernatant every other day for a week, changing the 0.1X MMR on the intervening day.
- After one week in the 100 mm Petri dish, move animals to half gallon tanks filled with frog water [0.5 g/L instant ocean and 2 mM Sodium phosphate dibasic (Na2HPO4: mw 141.96) pH 6.8]. Feed them as in step 2.4, but change water only 2-3 times per week when water is cloudy. Maintain the animals in a 12 hr light/12 dark light cycle with the lights turning on at 6 am.
- Place the half-gallon tank with the test subjects on a white surface like a lab bench underpad, at least 12 hr O/N, prior to testing.
- Test tadpoles at stages 45 to 50 as described in step 3 below.
2. Pre-behavior Setup: Equipment
- Set aside an area of the lab for the behavior assay. It should be in a quiet, low-traffic area with standard fluorescent lighting.
- Prepare the testing tanks for the animals.
The nested, half-gallon testing tanks are composed of two parts: an inner tank that will hold the water and tadpoles; and an outer tank with the visual stimulus.- For the inner tank, make a small mark on the outside corners with a sharpie, 5 cm from the bottom; this is the water line (Figure 1A, green dotted line).
- Also, for the inner tank, fill the divets in the tank (at the corners and the center) with an inert compound, like Sylgard elastomer. NOTE: The tadpoles will linger in these areas if they are not filled in.
- For the outer tank, cover exactly one half of the outside of one tank with black electrical tape and the other half with the white tissue paper Figure 1B.
- Place the tanks on the inoculating turntable.
- Setup the webcam/tripod so that it is above the testing tanks. Adjust the camera to allow visualization of the testing area, as shown in Figure 1C.
- Connect the webcam to a computer with QuickTime Player software installed5. Turn on the camera. In QuickTime Player, under File, select ‘New Movie Recording.’ The behavior assay setup will be visible on the computer right away.
- Drape a lightweight cotton cloth over the entire setup to reduce external cues that could affect tadpole behavior, as well as, reduce reflected light on the surface of the water.
- Ensure that the luminance under the cloth measures between 35-50 cd/m2.
3. Behavior Assay
- Fill the inner testing tank to the 5 cm water mark made in step 1.2.1 with frog water.
- Using a small net, gently move the animal into the inner testing tank.
- Open QuickTime software on computer and, using the outer tank as a guide, make sure that the camera is able to visualize and record the testing area.
- Write the animal name, date and time on a piece of paper and record with the camera.
- Place the test tank into the outer tank with the black side of the tank to the right Figure 2.
- Start recording the movie immediately after arranging the tank and set timer for 2 min.
- When the timer beeps, remove the inner test tank, rotate the outer tank 180° and place the inner test tank inside the outer tank. Start the timer. Note: the black side should now be on the other side.
- Repeat step 3.7, eight more times for a total of ten trials. Use Figure 2 as a guide, checking off each trial.
- Repeat the behavior assay on two separate days.
4. Retinal Axotomy
- Place animal in 0.02% tricaine until they are unresponsive to a tail pinch with a #3 forceps.
- Melt 1% agarose in 0.1X MMR and then add to a 60 mm Petri dish. Once cooled, make a small rectangular divot in the agarose and add the tadpole to the divot with a little 0.02% tricaine, so that the animal is partially submerged in the liquid.
- Pierce the skin at a 45 degree angle behind the dorsal region of the eye with a 25 G needle, while bracing the animal against forceps on the opposite side.
- Taking care not to snip the vein, which lies next to the optic nerve, carefully reach into the hole with #5 forceps, snip the optic nerve and flip it out of the way. If there is severe bleeding due to accidently snipping the artery, then quickly place the animal in 2% tricaine to euthanize. CAUTION: 2% tricaine solution can cause numbness in humans. This solution should be handled with gloves.
- To recover the animal after surgery, place animal in 100 mm Petri dish with 0.7X MMR and 50 µg/ml gentamicin for 20 min. Next, transfer tadpole to recovery tank with frog water. Let animal rest O/N.
- The next morning, test the visual function as described above (steps 3.1-3.9).
5. Analyze Results
- After the trials are finished, measure the amount of time the tadpole stays on the black side of the tank.
- Manually view the videos using software that displays the time and allows frequent pausing. Look at the video display time. Write down the start of the two-min trial, which is when the inner tank successfully nests inside the outer tank and is squared in view of the camera.
- Use the position of the tadpole’s eyes to define which side of the tank the animals are swimming on. Define crossing over from one side of the tank to the other only when both eyes have crossed the black/white line.
- Write down the beginning and end of each interval (in sec) that the animal spends on the black side. Pause and rewind the video to ensure accuracy. Total the sec within each trial.
- Input these numbers into the attached Behavior Worksheet. The worksheet will calculate the ratio of time that the animal spent on the white side by subtracting the time spent on the black side of the tank from the total 120 sec trial and divide by the total time in sec [(120 – total seconds spent on the black side)/120 sec]. The worksheet averages this ratio over all 10 trials.
- Use a student’s T-test, paired, two-tailed distribution to determine if the trials between days are significant (P ≤ 0.05).
Representative Results
Previous reports have shown that premetamorphic Xenopus tadpoles prefer to swim on the white side of a black/white tank and called this assay, the Background Color Preference Assay3 We have changed this assay in order to test the visual function of either eye of tadpoles in less than a week. In this way, their eyes can be collected for histological examination.
We show here how the response is due to visual cues. In Movie 1, the tadpoles in the left and right panels are the same animals. On the left are the animals before surgery and on the right, the animals after severing both optic nerves. We swirled the water to show that nothing is added to repel the animals from the black side or attract them to the white side. In the movie, we provide a schematic of the tadpoles in the bottom corners, which shows the state of the optic nerve for each set of animals. In Movie 1, the normal, un-operated tadpoles respond to background color in less than 30 sec in the test tank while the blind tadpoles swim aimlessly around the tank. The speed of the movies was increased and the actual time recorded in the upper right-hand corner.
To test the vision of one eye, we have included the results from an animal whose optic nerve was severed in one eye and tested in our assay. The optic nerve in the other eye was severed after two days of testing, and the vision-guided behavior tested on the following days again. In Figure 3, a graph shows the amount of time the tadpole chose to swim on the black side of the tank. This was measured on six consecutive days Figure 3A. As described above, the right optic nerve was severed after behavior was tested on day 2 and the left, after behavior test on day 4. The tadpole preferred the white side of the tank randomly at 50% of the time Figure 3C. Notice that the animal spent more time on the white side of the tank during the last 6 trials on days 1 through 4. The amount of time spent on the black side was more even on days 5 and 6, when the animal was blind in both eyes. The results from each of the two days were averaged together and presented in a graph form Figure 3C.

Figure 1. Experimental set-up of vision-guided behavior assay. (A) The inner test tank remains transparent and is marked to indicate water levels (green dashed lines at the corner). (B) The outer test tank is covered in black electrical tape on one half and white tissue paper on the other. (C) The tadpoles are visualized using a webcam placed on a tripod and connected to a portable computer. The testing tanks rest on an inoculating turntable to allow for easy turning between trials. Please click here to view a larger version of this figure.

Figure 2. Schematic of how the vision-guided behavior assay is performed. The rectangles represent the test tank, showing how the half white/half black area changes with each trial. This can be used during the behavior assay to mark the trial number. The test tank on the right is a close-up of the tadpole and its expected behavior during the two min assay. Please click here to view a larger version of this figure.
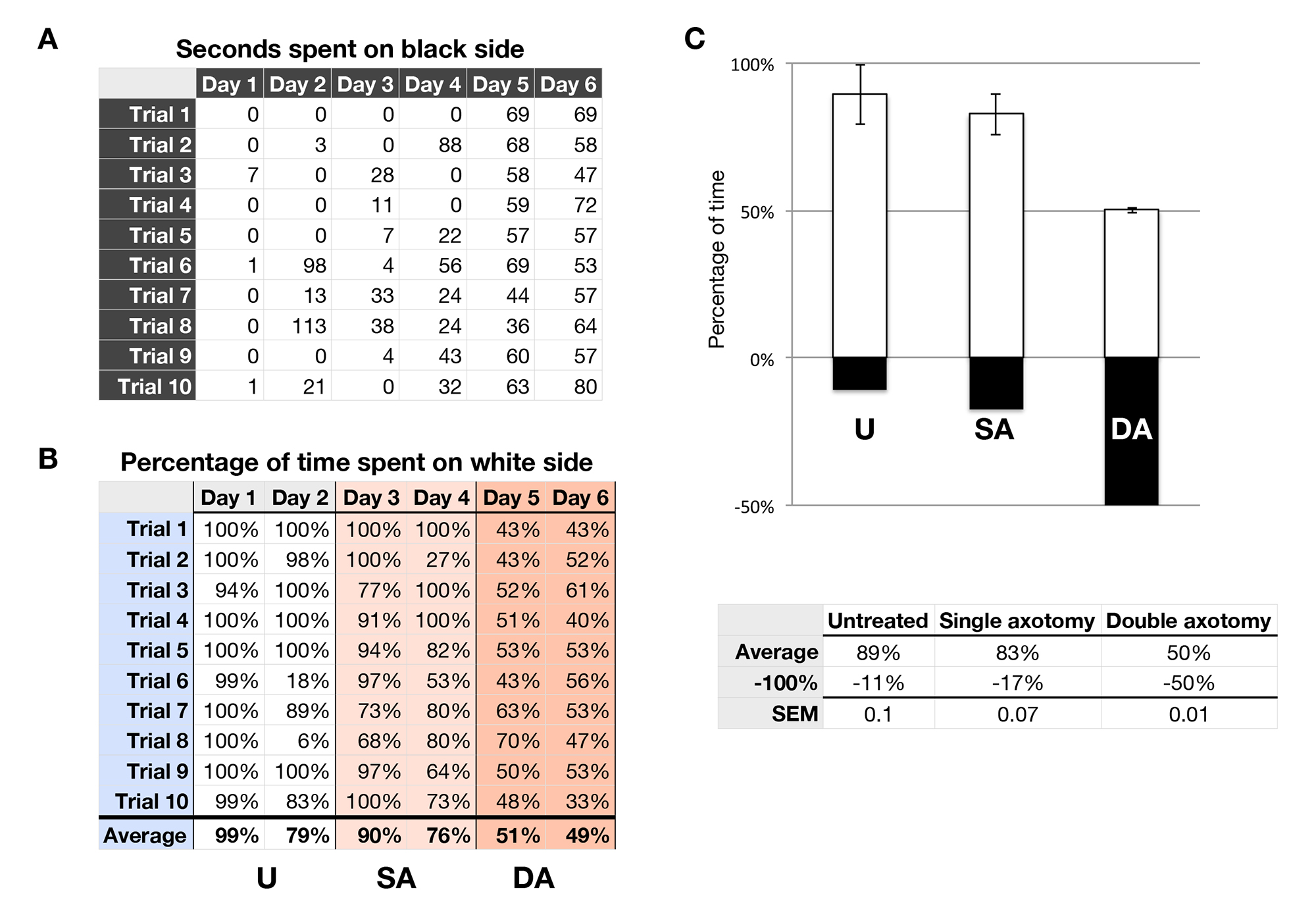
Figure 3. A representative experiment tallying the percentage of time the tadpole swam on the white side of the test tank. (A) The amount of sec the tadpole spent on the black side of the tank is measured and placed in a table. (B) The table in A is converted into the percentage of time spent of the white side of the tank. This is done by subtracting the sec spent on the black side from the total trial time (120 sec) and dividing by 120 sec. Tadpoles that were untreated (U), had a single axotomy (SA) and then a double axotomy (DA) were tested. (C) The graph represents the average percentage of time spent in the white side (white bar) and black side (black bar). The table used to make the graph is shown below. Please click here to view a larger version of this figure.
Movie 1. Behavioral response of sighted and blind animals in the white background preference assay. Tadpoles in the left and right panels are the same, but the ones on the right have undergone double retinal axotomy. A schematic in the bottom corners indicates whether the optic nerve is intact or severed (blue bar). The timer in the upper right hand corner indicates time. The movie is sped up for easier viewing. Once in the white area, notice how the tadpoles swim close to the boundary between white and black and reverse direction. Please click here to view this video.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| Trial 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ratio of time spent on white side | ||||||
| Ratio 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 7 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 8 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 9 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ratio 10 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Average | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| Trial 1 | 0 | 0 | 0 | 0 | 69 | 69 |
| Trial 2 | 0 | 3 | 0 | 88 | 68 | 58 |
| Trial 3 | 7 | 0 | 28 | 0 | 58 | 47 |
| Trial 4 | 0 | 0 | 11 | 0 | 59 | 72 |
| Trial 5 | 0 | 0 | 7 | 22 | 57 | 57 |
| Trial 6 | 1 | 98 | 4 | 56 | 69 | 53 |
| Trial 7 | 0 | 13 | 33 | 24 | 44 | 57 |
| Trial 8 | 0 | 113 | 38 | 24 | 36 | 64 |
| Trial 9 | 0 | 0 | 4 | 43 | 60 | 57 |
| Trial 10 | 1 | 21 | 0 | 32 | 63 | 80 |
| Ratio of time spent on white side | ||||||
| Ratio 1 | 100% | 100% | 100% | 100% | 43% | 43% |
| Ratio 2 | 100% | 98% | 100% | 27% | 43% | 52% |
| Ratio 3 | 94% | 100% | 77% | 100% | 52% | 61% |
| Ratio 4 | 100% | 100% | 91% | 100% | 51% | 40% |
| Ratio 5 | 100% | 100% | 94% | 82% | 53% | 53% |
| Ratio 6 | 99% | 18% | 97% | 53% | 43% | 56% |
| Ratio 7 | 100% | 89% | 73% | 80% | 63% | 53% |
| Ratio 8 | 100% | 6% | 68% | 80% | 70% | 47% |
| Ratio 9 | 100% | 100% | 97% | 64% | 50% | 53% |
| Ratio 10 | 99% | 83% | 100% | 73% | 48% | 33% |
| Average | 99% | 79% | 90% | 76% | 51% | 49% |
Worksheet 1. White background preference assay worksheet. This worksheet has the calculations built in so that it can be used to enter numbers and tally results.
Discussion
We report here a simple vision-guided behavioral assay that can be easily performed in under a week by minimally trained lab personnel. While other assays require specialized equipment and expertise in animal behavior, this assay allows a quick test to determine visual function. Another behavioral assay, the Visual Avoidance assay, has been developed to determine how the tectum contributes to visual perception in Xenopus10. This assay measures spatial tuning and contrast sensitivity in response to a moving versus stable background, and thus, can measure more specialized vision-based behavior than the simple assay reported here. An automated behavior system has been reported that can measure the amount of time spent on one side of the tank8. There is also commercially available software (e.g. ANY-maze software) that has been used to measure the amount of time zebrafish spend in dark or bright environments9. The assay we report here does not require the purchase of these types of expensive equipment or software, but instead relies on everyday reagents and equipment to measure visual function.
The vision-guided assay is performed on one animal to test its visual function. When comparing the behavioral assay results of several tadpoles to one another, we observed no statistical difference between animals within treatment groups. We tested three tadpoles on six consecutive days and also tested another five animals on three consecutive days, running ten trials per day. In either case, we found no statistically significant changes among the untreated or single axotomy groups (P = 0.2067), yet obtained a statistically significant change when these animals were compared to the double axotomy group (P = 0.002). This was measured using Prism 6.0c software running a 2-way ANOVA statistical test with a Tukey’s multiple comparison test (n = 8 tadpoles). To have the maximal amount of data points before surgery, we suggest using the six-day behavior assay. If results need to be obtained quicker with less degeneration of the tissue, we suggest using the three-day system.
This assay tests one animal at a time. We performed pilot studies to see if two tadpoles or more could be measured simultaneously. To determine if having two tadpoles in one tank affected their preference for the white side of the tank, we tested each tadpole separately and then together. Since the images were taken above the tank, if the tadpoles swam above or below each other for any length of time, we found it difficult to tell them apart. If we used tadpoles of different sizes, we observed that the larger one would affect where the smaller one swam. Future technologies could be developed to genetically mark tadpoles, which would allow better tracking of multiple animals of the same size.
In performing this assay on dozens of tadpoles, we observed that some tadpoles preferred the white side more consistently than others. Tadpoles, whose behavior varied by more than 10% on two consecutive days, we would take were tested again on a third day before surgery. This may have been because of another observation: we noticed that placing the animals on a white background also made a difference in their preference for the white side of the tank. Having that extra day may have helped condition some tadpoles to prefer the white side of the tank. A limitation of this assay is that it only tests visual function. The variability we observed could be due to other developmental defects, besides reduced visual function or blindness. For this reason, animals that showed less than 60% preference before surgery were not tested further.
We also tested a range of ages of pre-metamorphic tadpoles. We found that tadpoles younger than stage 45 were very small and their eyes were difficult to track using our webcam. Older tadpoles (stages 51 – 55+) were more distracted; although they ultimately spent more time on the white side of the tank, they seem to take longer to swim there. These older animals were bigger, and therefore, easier to track, but needed testing prior to surgery as stated above. In contrast, the majority of tadpoles at stage 45 up to 50 behaved as expected and animals of these ages would be best to use in future studies.
Circadian light changes have been shown to affect vision-guided behavioral responses in Xenopus2. Consistent with this observation, we noticed that the same animal that quickly swam to the white side of the test tank in trials conducted in the morning, took much longer during afternoon behavior tests. After conducting a number of ‘same animal/different time’ trials, we determined the best time for the behavior assay was from 6 am to 1 pm.
We have also used a digital handheld camcorder connected by firewire to test animals under low light conditions5. This allowed testing of nighttime or scotopic vision, in which the animal uses its rod photoreceptors to see. Conditions were altered by simply finding the least amount of light required for the animal to perform the vision-guided assay. The assay presented here tests photopic vision, in which the animal mostly uses its cone photoreceptors to see. By combining these approaches, both types of vision, scotopic and photopic, can be tested.
We used this assay to test the visual function of animals with an ectopic eye grown from pluripotent cells overexpressing the intrinsically expressed eye field transcription factors and the extrinsic factor, Noggin. Eyes generated from these tissues were functional4,11. Noggin also plays a role in anterior central nervous system development12 but using this vision-guided assay in conjunction with the animal cap transplant assay, its role in eye development was uncovered. Other extrinsic factors that affect early embryo formation but have not yet been shown to have a role in eye development may induced retinal progenitor formation in pluripotent animal cap cells4,11. Using this behavioral assay, future studies could determine the visual function of these newly generated eyes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by grants from the National Institutes of Health: EY015748, EY017964 (MEZ), and EY019517 (ASV). This work was also supported by the Research to Prevent Blindness Unrestricted Grant to the Ophthalmology Department and the Lions of Central New York. We would also like to thank our animal technician, Matthew Mellini, for his excellent care of the animals and for stepping in to star in this video.
Materials
| 1/2-gal. Flex-Tank with Cover | eNasco | SB19271M | size: 5-3/8" x 7" x 3-3/4" |
| Black electrical tape | |||
| White tissue paper | |||
| Large Inoculating Turntable | VWR | 50809-022 | size: Dia 114.3 x H 76.2 mm (4 1/2 x 3") |
| Durasorb Underpad | VWR | 82004-836 | size: 43.2 x 60.1 cm (17 x 24") |
| Kim wipe | Krackeler Scientific, Inc. | 1945-34155-CS | |
| Standard Tripod | various | ||
| iSight camera or webcam | Apple | M8817LL/A | Good for larger tadpoles but small ones are difficult to see |
| Portable computer | Apple/PC | various | We used a 13" MacBook, 2 GHz Intel Core 2 Duo running MacOSX Lion 10.7.5 |
| MiniDV Handycam Camcorder | SONY | DCR-HC42 | Connected by firewire to the computer with a 6-conductor and 4-conductor alpha FireWire 400 |
| Handycam Station | SONY | DCRA-C121 | This can be used for connecting firewire to camera |
| QuickTime Player software | Quicktime | Version 10.1 | |
| 26 Gauge Needle (5/8" length) | VWR | BD305115 | |
| Dumont #5 Forceps Biology | Fine Science Tools | 11295-10 | |
| Disposables | |||
| Gentamicin Sulfate [50 mg/ml] | Fisher Scientific | 17-528Z | Stored at room temperature |
| Sylgard 184 Silicone Elastomer | Fisher Scientific | NC9644388 | |
| Instant Ocean | Doctors Foster and Smith | CD-116528 | Stock solution = 100 g/L stored at room temperature |
| Sodium phosphate dibasic | Sigma Aldrich | S0876 | Stock solution = 0.4 M stored at room temperature |
| Tricaine (Ethyl-3-aminobenzoate methanesulfonate salt) | Sigma Aldrich | A5040 | Stock solution = 10% stored at -20˚C |
| In vitro fertilized embryos | eNasco | LM00490MX | 100 embryos/unit |
References
- Maurer, C. M., Huang, Y. Y., Neuhauss, S. C. Application of zebrafish oculomotor behavior to model human disorders. Rev Neurosci. 22 (1), 5-16 (2011).
- Solessio, E., Scheraga, D., Engbretson, G. A., Knox, B. E., Barlow, R. B. Circadian modulation of temporal properties of the rod pathway in larval Xenopus. J Neurophysiol. 92 (5), 2672-2684 (2004).
- Moriya, T., Kito, K., Miyashita, Y., Asami, K. Preference for background color of the Xenopus laevis tadpole. J Exp Zool. 276 (5), 335-344 (1996).
- Viczian, A. S., Solessio, E. C., Lyou, Y., Zuber, M. E. Generation of functional eyes from pluripotent cells. PLoS Biol. 7 (8), (2009).
- Choi, R. Y., et al. Cone degeneration following rod ablation in a reversible model of retinal degeneration. Invest Ophthalmol Vis Sci. 52 (1), 364-373 (2011).
- Viczian, A. S., Zuber, M. E. Tissue determination using the animal cap transplant (ACT) assay in Xenopus laevis. J Vis Exp. 16 (39), (2010).
- Nieuwkoop, P. D., Faber, J. . Normal table of Xenopus laevis (Daudin) : a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. , (1994).
- Blackiston, D., Shomrat, T., Nicolas, C. L., Granata, C., Levin, M. A second-generation device for automated training and quantitative behavior analyses of molecularly-tractable model organisms. PLoS One. 5 (12), (2010).
- Rosemberg, D. B., et al. Differences in spatio-temporal behavior of zebrafish in the open tank paradigm after a short-period confinement into dark and bright environments. PLoS One. 6 (5), (2011).
- Dong, W., et al. Visual Avoidance in Xenopus Tadpoles is Correlated With the Maturation of Visual Responses in the Optic Tectum. J Neurophysiol. 101 (2), 803-815 (2009).
- Lan, L., et al. Noggin Elicits Retinal Fate In Xenopus Animal Cap Embryonic Stem Cells. Stem Cells. 27 (9), 2146-2152 (2009).
- De Robertis, E. M., Kuroda, H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 20, 285-308 (2004).

