ERRATUM NOTICE
Important: There has been an erratum issued for this article. Read more …
Summary
介绍了一种制备聚 (五氟丙烯酸五氟苯基丙烯酸酯) (聚聚丙烯) 接枝二氧化硅微珠的制备方案。然后用抗体固定化聚多聚体 (pfpa) 功能化表面, 并通过免疫沉淀成功地用于蛋白质分离。
Abstract
我们演示了一种制备聚 (五氟苯基丙烯酸酯) (聚 (pfpa) 接枝二氧化硅珠用于抗体固定化和随后免疫沉淀 (ip) 应用的简单方法。聚 (pfpa) 接枝表面是通过一个简单的两步工艺制备的。第一步, 3-氨基丙基三乙氧基硅烷 (aptes) 作为链接分子沉积在二氧化硅表面。在第二步中, 通过可逆添加和碎裂链转移 (raft) 聚合合成的聚 (ppa) 均聚物通过五氟苯基 (pfp) 单元在聚合物和 aptes 上的胺基团。通过 x 射线光电子能谱 (xps) 证实了 aptes 和聚 (ppa) 在二氧化硅颗粒上的沉积, 并通过动态光散射 (dls) 测量的粒径变化进行监测。为了提高珠子的表面亲水性, 还采用氨基化聚乙二醇 (氨基 peg) 部分取代了聚 (pfpa)。然后用抗体固定化聚乙二醇 (pfpa) 接枝二氧化硅微珠进行 ip 应用。为了进行演示, 采用了抗蛋白激酶 rna 激活 (pkr) 的抗体, ip 效率是由西方印迹决定的。分析结果表明, 抗体固定化珠确实可以用来丰富 pkr, 而非特异性蛋白质相互作用最小。
Introduction
活性聚合物刷子近年来受到了广泛的关注。它们可用于固定在有机或无机材料上的功能分子, 以创建在检测和分离等领域的活化表面, 如检测和分离1、2、3、4、 5。在所报告的反应聚合物中, 含有五氟苯基酯单位的聚合物特别有用, 因为它们与胺的反应性很高, 对水解有抵抗力, 6。其中一种聚合物是聚 (pfpa), 它可以很容易地功能化后与分子含有一级或二级胺 7,8, 9,10。在一个例子中, 聚 (pfpa) 刷与氨基螺旋体反应, 以创建光反应表面7。
聚 (ppa) 的制备及其应用已在以前的一些出版物6、7、8、9、10、11、12中介绍 ,13,14,15,16,17。特别是, theato 和他们的同事报告了通过 "嫁接到" 和 "嫁接到" 方法7、8、10、11、12合成聚 (pfpa) 刷的情况。.在 "接枝到" 方法中, 合成了聚 (甲基硅基氧烷)-聚 (五氟苯基丙烯酸酯) (聚 (mssq-ppa)) 混合聚合物 8,10,11, 12.聚 (mssq) 组分能够与许多不同的有机和无机表面形成强烈的粘附, 从而使聚 (pfpa) 成分在涂层材料表面形成画笔层。在 "接枝自" 方法中, 采用表面启动可逆加法和碎裂链转移 (si-raft) 聚合制备聚 (pfpa) 刷7。在这种情况下, 表面固定链转移剂 (si-cta) 首先通过硅硅烷反应共价连接到基板上。固定化的 si-cta 随后参与了 pfpa 单体的 si-raft 聚合, 产生了与基材有稳定共价连接的密集填充聚 (pfpa) 刷子。
利用通过 si-raft 聚合合成的聚 (pfpa) 刷, 我们最近证明了聚接枝二氧化硅颗粒上抗体的固定化及其随后在蛋白质纯化中的应用 18.使用聚 (pfpa) 刷进行抗体固定被发现可以解决一些与当前通过 ip 分离蛋白质相关的问题。传统的 ip 依靠使用蛋白阿比作为抗体固定的链接器 19,20,21。由于使用蛋白 a 可以使抗体具有特定的方向, 因此实现了高目标抗原的恢复效率。然而, 蛋白 a 的使用存在着非特异性蛋白质相互作用以及蛋白质恢复过程中抗体的丢失, 这两者都导致了高水平的背景噪声。为了解决这些缺点, 已经探索了抗体与固体支持的直接交联。由于交联抗体的随机方向, 这类技术的效率通常很低。对于聚 (pfpa) 接枝底物, 抗体的固定化是永久性的, 是通过 pfp 装置和胺功能对抗体的交换反应来实现的。虽然抗体取向仍然是随机的, 但该系统受益于有许多反应性的 pfp 位点, 可通过聚合程度来控制。此外, 我们还表明, 通过用氨基 peg 部分取代 pfp 单元, 可以调整表面亲水性, 进一步提高系统18的蛋白质回收效率.总体而言, 聚 (pfpa) 接枝二氧化硅颗粒被证明是传统 ip 的有效替代品, 具有合理的效率和更清洁的背景。
在这篇贡献中, 我们报告了一种替代方法, 用于制备用于抗体固定和 ip 应用的聚 (pfpa) 接枝表面。在一个简单的两步过程中, 如图 1所示, aptes 链接器分子首先沉积在二氧化硅表面, 然后聚 (ppa) 聚合物通过 pfp 单元之间的反应共价地附着在链接器分子上。聚合物和胺功能在 aptes 上。这种制备方法允许聚 (pfpa) 与基材表面永久交联, 但避免了与超金属-cta 合成和聚刷子 si-raft 聚合相关的许多并发症。仍然可以部分用氨基 peg 取代私营部门筹资和伙伴关系方案装置, 从而能够对聚合物刷子表面特性进行微调。我们表明, 这样制备的聚 (pfpa) 接枝二氧化硅珠可以用抗体固定化, 并用于通过 ip 浓缩蛋白质。本文详细介绍了珠子制备过程、抗体固定和 ip 检测, 供有兴趣寻求替代传统的基于蛋白质 a 的 ip 的读者使用。
Subscription Required. Please recommend JoVE to your librarian.
Protocol
1. 制备聚 (ppa) 共聚物
- aibn 的再结晶
- 将5克 2, 2 '-azobis(2 甲基丙腈) 与25毫升甲醇在250毫升烧杯中混合。将烧杯浸入60°c 的油浴中, 然后用搅拌棒大力搅拌混合物, 直到 aibn 完全溶解。
- 通过滤纸 (5-8μm 颗粒保留) 过滤温溶液, 并将滤液储存在 4°c, 使晶体慢慢形成。
- 通过过滤收集再结晶的 aibn。将收集到的产品与25毫升的新鲜甲醇混合, 重复再结晶过程。
- 在室温 (rt) 下, 在真空烤箱中擦干2倍再结晶的 aibn。将产品存放在黑暗中 & lt;-10°c。
- 二硫代苯甲酯的合成25
- 准备一个500毫升的三颈圆底烧瓶, 配备了一个磁搅拌杆, 回流冷凝器, 一个下降漏斗, 和橡胶隔膜。通过回流冷凝器将烧瓶连接到氮气管道, 并用氮气冲洗内部空气。通过隔膜插入温度计。通过相同的隔膜在四氢呋喃 (thf) 中加入41毫升 (0.041 mol) 的苯基溴化镁 1 m 溶液。
- 在油浴中将苯基溴化镁溶液加热至40°c。然后通过落漏斗缓慢加入 3.1 g (0.041 摩尔) 的二硫化碳, 将溶液温度保持在40°c。
- 通过超过15分钟的落漏斗, 在合成的混合物中加入7.1 克 (0.042 摩尔) 的溴化氢, 将反应温度提高到50°c。在这个温度下继续搅拌45分钟。
- 将反应混合物转移到分离漏斗中, 用15毫升的冰冷水稀释。加入15毫升的二乙醚提取产品, 去除较低的水层。再重复两次用乙醚提取。
- 用大量的水清洗有机相, 然后用盐水 (水中 50% (w/v) 的氯化钠溶液) 清洗, 并在无水硫酸镁上干燥产品。
- 使用旋转蒸发器在35°c 的真空中取出溶剂。
- 以硅胶 (孔径60、63-200 目粒径) 和石油醚为洗脱剂, 以5克二硫代苯甲酸苯 (bdb) 为红油, 采用柱层析法纯化产品。通过1h 核磁共振 (400 mhz, cdcl3) 确认产品纯度: 8.02-7.99 (2h, m), 7.55-7.50 (1h, m), 7.41-7.29 (7h, m), 4.60 (2h, s)。
- raft 聚合9,26合成聚 (pfpa)
- 市售全氟化二酚 pa 单体含有少量抑制剂。聚合前, 通过用基本氧化铝包装的一次性注射器将抑制剂通过单体取出。
- 加入0.4 毫克 (0.0024 mmol) 的再结晶 aibn, 4.3 毫克 (0.018 mmol) 的 bdb, 1012 毫克 (4.25 mmol) 的无抑制性 ppa, 并将 0.7 ml 的无水单肠酸酯添加到20毫升的 schlenk 询问中。
- 将烧瓶连接到 schlenk 线, 用至少三个冻水-解冻循环对混合物进行去气体处理。简单地说, 将反应混合物冷冻在液氮浴中。应用真空去除头部空间中的气体。密封烧瓶, 然后从液氮中取出, 使含量在 rt 解冻。
- 将烧瓶放在70°c 的油浴中, 并在n2清洗下反应4小时。
- 要终止反应, 请从油浴中取出烧瓶, 并将反应内容暴露在空气中。
- 将聚合物沉淀在冷甲醇中, 然后在40°c 的真空烤箱中干燥回收的聚合物。
- 在聚合物分子量测量中, 采用凝胶渗透色谱法 (gpc)。采用 thf 作为35°c 的流动相, 流量为 1 mL/min, 并使用单分散聚苯乙烯标准构建校准曲线。为了获得 gpc 测量, 将聚合物溶解在 thf (1-2 mg/ml) 中, 并通过0.2μm 一次性聚四氟乙烯 (ptfe) 过滤器进行过滤。将样品的100μl 注入 gpc 仪器。使用聚苯乙烯校准曲线将测量样品保留时间转换为分子量。
2. 制备聚 (ppa) 功能化 sio2 珠
- aptes 治疗 sio2 珠
- sio2 颗粒以 5% (w/v) 水悬浮液的形式提供.将 sio2 悬浮液的 0.8 毫升与40毫克的 aptes 和8毫升的甲醇结合在一个配备了搅拌棒的20毫升闪烁小瓶中。
- 允许反应在 rt 进行5小时与剧烈搅拌。
- 将溶液转移到锥形管。为了分离 aptes 功能化的 sio2 珠 , 以 10, 000 x g 离心溶液 5分钟, 然后去除上清液。将珠子重新分散在3毫升的新鲜甲醇中, 将其清洗干净。用手摇管进行混合, 但如有必要, 可在水浴中通过超声效果提高分散性几秒钟。以 10, 000 x g 离心珠子 5分钟, 取出上清液, 再重复一次清洗步骤。
- 将甲醇清洗后的二氧化硅 2 珠与3毫升的二甲基亚硫醚 (dmso) 结合使用。用手摇晃混合物, 或在必要时发出几秒钟的声音, 直到珠子完全分散在 dmso 中。以 10, 000 x g 离心珠子 5分钟, 然后取出上清液。重复此步骤, 确保从甲醇到 dmso 的完整溶剂交换。
注: 最终悬浮液包含 ates 功能化的 sio2 微珠, 分散在 dmso 的 4 ml 中。 - 要检查粒度分布, 请执行 dls 分析。采取在步骤2.1.4 准备的悬浮液的一滴, 并放置到一个一次性的紫外线试剂盒。用新鲜的 dmso 填充样品, 直到样品满了, 将样品稀释。将样品插入电池支架中, 开始数据采集。对于粒度测量, 请使用以下设置参数: 温度: 25°c;平衡时间: 120秒;测量持续时间: 自动。
- 要检查表面成分, 请执行 xps 分析。在40°c 的真空烤箱中, 在40°c 的真空烘箱中干燥在步骤2.1.4 中制备的悬浮液中的小样品。取干燥的聚合物, 均匀地包装在 0.5 cm x 0.5 cm 样品支架上。将样品加载到高真空室 (10-8 torr) 中, 并开始数据采集。对于所使用的特定 xps 仪器, 使用在15kv 和 6.7 ma 下工作的单色 al kV x 射线生成光电子, 并使用混合模式放大与分析仪在 50 ev 传递能量下收集高分辨率光谱和 100 ev 通能元素测量。
- 将聚聚 (pfpa) 接枝到 aptes 功能化的 sio2 珠中
- 在20毫升的闪烁小瓶中, 在2毫升的 dmso 中溶解20毫克的聚 (pfpa), 制备聚 (pfpa) 溶液。
注: 在本研究中, 使用了相对较低的分子量聚 (pfpa) (20 kg/mol)。因此, 尽管聚合物浓度较高 (10 mg/ml), 但没有观察到聚合物交联的证据。如果使用更高的分子量聚合物, 则可能需要调整聚合物溶液浓度, 以避免可能的交联。 - 在聚 (ppa) 溶液中加入1毫升悬浮在 dmso 中的 aptes 功能化 sio2 珠子 (从步骤 2.1.4)。在 rt 反应 1小时, 搅拌有力。
- 以 10, 000 x g 离心5分钟分离接枝的聚氧二微珠, 然后去除上清液。通过加入3毫升的 dmso 清洗珠子, 用手晃动或几秒钟的超声混合。以 10, 000 x g 离心珠子 5分钟, 然后取出上清液。用 dmso 对聚 (pfpa) 接枝的 sio2 珠子重复清洗两次。
- 用三重蒸馏水 (tdw) 再清洗两次珠子。在这一步中, 将珠子与 tdw 的3毫升结合起来, 然后用手晃动或几秒钟的超声。以 10, 000 x g 离心珠子 5分钟, 然后取出上清液。
- 若要检查粒度分布, 请按照步骤2.1.5 中描述的过程执行 dls。要检查表面化学, 请按照步骤2.1.6 中描述的步骤执行 xps。
- 在20毫升的闪烁小瓶中, 在2毫升的 dmso 中溶解20毫克的聚 (pfpa), 制备聚 (pfpa) 溶液。
3. 用 pfpa 接枝的 sio2珠的制备
- 制备聚 (pfpa) 溶液, 在20毫升的闪烁小瓶中, 将20毫克的聚 (pfpa) 溶解在2毫升的 dmso 中。
- 制备 peg 溶液时, 将氨基功能化的聚乙二醇溶于 dmso 1 毫升。使用的 peg 的确切数量由所需的私营部门筹资和伙伴关系司替代程度决定, 由下面所示的公式确定:
氨基聚乙二醇 (g/聚乙二醇 (pfpa)) = (n _ poly (pfpa) xx (mw _ peg/w _ poly (pfpa))
其中 n _ 聚 (pfpa) = 聚 (pfpa) 聚合度
% peg-sub =% peg 替代
mw _ peg = 氨基聚乙二醇分子量
mw _ poly (pfpa) = 聚多分子量 (pfpa) - 将 peg 溶液转移到聚 (pfpa) 溶液中。在 rt 反应 1小时, 搅拌有力。
- 要准备挂在 dmso 中的 apt功能化 sio2 磁珠, 请按照步骤2.1 中所示的相同步骤操作。将珠子悬浮液的1毫升转移到步骤3.3 中制备的 peg 取代聚 (pfpa) 溶液中。允许聚 (pfpa) 和 aptes 功能化的 sio2 珠子之间的接枝在 rt 进行 1小时, 并进行剧烈搅拌。
- 以 10, 000 x g 离心5分钟分离珠子, 然后去除上清液。通过加入3毫升的 dmso 清洗珠子, 用手晃动或几秒钟的超声混合。以 10, 000 x g 离心珠子 5分钟, 然后取出上清液。重复 dmso 清洗两次。
- 用 tdw 再清洗两次珠子。在此步骤中, 将珠子与 3ml tdw 组合, 然后通过与手晃动或几秒钟的超声操作进行混合。以 10, 000 x g 离心珠子 5分钟, 然后取出上清液。
- 在真空烤箱中隔夜在40°c 下擦干珠子。
4. 聚极 (pfpa) 接枝 sio2珠的抗体固定
注: 无论聚 (pfpa) 上的 peg 取代百分比是什么, 都使用相同的程序。通过在 tdw 中溶解 pbs 片制备磷酸盐缓冲盐水 (pbs)。在 pbs 中加入半1000丁-20 (pbst), 制备0.1% 的磷酸盐缓冲盐水。
- 在 1.5 ml 微离心管中加入5毫克的聚 (pfpa) 接枝的 sio 2 珠子.
- 加入800μl 的 pbs 清洗珠子, 并通过涡流进行混合。在 rt 以 10, 000 x g 的速度离心珠子, 取出上清液, 重复三次清洗步骤。
- 加入350μl 新鲜 pbs, 50μl 0.1% (v/v) pbst, 6.67 微克抗体。在4°c 的旋转器上孵化 ~ 20小时。
- 将珠子清洗以去除未绑定的抗体。以 400 x g 和4°c 离心珠子 1分钟, 取出上清液, 小心加入400μl 的裂解缓冲液。通过向上和向下移液五次轻轻重新悬浮珠子。
注: 用于清洗珠子的裂解缓冲液应与细胞裂解和 ip 过程中使用的缓冲液相同, 只是添加二硫醇和蛋白酶抑制剂是可选的 (请参阅步骤 5)。 - 重复此清洗步骤三次。最后清洗后, 尽可能地取出上清液。
5. 细胞裂解和免疫沉淀
- 裂解缓冲液和洗涤缓冲液的制备
- 准备裂解缓冲液 (50 mm Tris-HCl (ph 8.0)、100 mm kcl、0.5% (v/v) np-40、10% (v/v) 甘油、1 mm 二硫基三醇 (dtt) 和蛋白酶抑制剂鸡尾酒)。
- 准备洗涤缓冲液 (50 mm Tris-HCl (ph 值 8.0)、100 mm kcl、0.1% (v/v) np-40 和 10% (v/v) 甘油)。
- 将缓冲液存放在4°c。
- 细胞的制备
- 在 ip 实验前一两天对细胞 (hela 细胞) 进行播种, 并在37°c 和 5% co 2 下生长细胞。
- 收集约 1.4 x 10 7 细胞与细胞刮刀和转移到一个15毫升锥形管。在 rt 以 380 x 克的速度离心细胞 3分钟, 取出上清液, 用1毫升的冷 pbs 重新悬浮, 转移到 1.5 ml 的微离心管中。
- 在4°c 条件下, 以 10, 000 x g 的温度离心细胞 30秒. 将上清液干净地取出。去除上清液后, 细胞颗粒可储存在-80°c。
- 细胞裂解物的制备
- 用400μl 的裂解缓冲液重新悬浮细胞颗粒。用超声波机对细胞进行治疗。
- 超声后, 短暂旋转, 在4°c 下离心裂解液在 20, 000 x 克, 10分钟。
- 将上清液转移到新的 1.5 ml 离心管中。
- 免疫沉淀
- 将300μl 细胞裂解液转移到先前制备的抗体孵育聚 (pfpa) 接枝的 sio2微珠中。将细胞裂解液保留30μl 作为输入样品, 保存在新的微离心管中。将输入样品存放在4°c。
注: 细胞裂解液中的蛋白质总量应在4毫克左右。 - 在4°c 的旋转器上将裂解珠混合物培养3小时。
- 在4°c 下以 400 x g 离心混合物 1分钟, 取出上清液, 小心加入400μl 洗涤缓冲液。通过向上和向下移液约五次, 轻轻重新悬浮珠子。
- 重复此清洗步骤三次。最后清洗后, 尽可能地取出上清液。
- 制备2x 十二烷基硫酸钠 (sds) 负载染料 (25% (v/v) 甘油、0.1% (w/v) 溴苯酚蓝 (bpb)、60 mm Tris-HCl (ph 6.8%)、2% (w/v) sds 和 2.75 mm 2-硫醇)。将 2x sds 加载染料存放在-20°c。在珠子和储存的输入样品中加入30μl 的 2x sds 加载染料, 并在95°c 下加热10分钟。
- 加热后, 使用西方印迹27对样品进行分析, 或将样品存放在-20°c。
- 将300μl 细胞裂解液转移到先前制备的抗体孵育聚 (pfpa) 接枝的 sio2微珠中。将细胞裂解液保留30μl 作为输入样品, 保存在新的微离心管中。将输入样品存放在4°c。
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
图 1显示了制备聚 (pfpa) 接枝 sio2珠的原理图, 无论是否有 peg 取代。为了监测 aptes 和聚 (ppa) 接枝过程, 裸露的 sio2珠子、aptes 功能化的 sio2珠子和聚 (pfpa) 接枝的 sio2 珠子都以 dls (图 2) 和 xps (图 3) 为特征。珠子的 ip 效率是由西方印迹决定的。图 4显示了西方 ip 的印迹结果, 使用 1% peg 取代聚聚 (pfpa) 接枝微珠, 其中的珠子是在没有抗体、非特异性抗体或抗 pkr 抗体的情况下孵育的。图 5显示了西方的 ip 印迹结果, 使用 0% peg 取代聚 (pfpa) 接枝珠和 1% peg 取代聚聚 (pfpa) 接枝珠, 两者都用抗 pkr 抗体孵育。

图 1: 使用 aptes 作为链接分子的聚 (pfpa) 接枝 sio2珠的制备原理图。(a) 聚 (ppa) 接枝珠。(b) 部分 peg 取代聚醚 (pfpa) 接枝珠。请点击这里查看此图的较大版本.

图 2: dls 测量 (a) 裸露的 sio2珠子 (sio2), (b) aptes 功能化的 sio2珠子 (aptes-sio2), (c) 多聚 sio 2 微珠 (聚 (ppa) -sio2), 分散在 dmso 中.报告了每个样品的 z 平均直径 (d) 和多分散性指数 (pdi)。请点击这里查看此图的较大版本.

图3:15 型 sio2微珠 (sio2)、aptes 功能化 sio 2 微珠 (aptes-sio2) 和聚 (pfpa) 接枝的 sio2微珠 (聚 (pfpa)-sio 2) 的 xps 光谱.所检查的峰对应于 (a) si 2p、(b) o 1s、(c) n 1s 和 (d) f 1s。请点击这里查看此图的较大版本.

图 4: 使用 1% peg 取代聚 (pfpa) 接枝微珠处理的 1% peg 取代聚多 (pfpa) 接枝微珠的西方印迹结果, 使用无抗体 (车道 2)、非特异性抗体混合物、正常兔 igg (车道 3) 或抗 pkr 抗体 (车道 4) 进行处理.车道1显示 ip 之前的输入蛋白混合物。请点击这里查看此图的较大版本.

图 5: 使用 0% peg 取代聚 (pfpa) 接枝珠 (车道 2) 和 1% peg-取代聚多边形 (车道 3) 的 ip 的西方印迹结果, 两者均使用抗 pkr 抗体进行处理.车道1显示 ip 之前的输入蛋白混合物。请点击这里查看此图的较大版本.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
聚 (pfpa) 接枝 sio2 珠的合成如图 1所示。通过使用 aptes 作为链接分子, 聚 (pfpa) 刷共价接枝到 sio2 基板可以通过一个简单的两步过程制备。虽然一些私营部门筹资和伙伴关系司单位被牺牲用于与 aptes 的反应, 但预计大量私营部门筹资和伙伴关系方案单位将继续可用, 以便以后与氨基-peg 或抗体发生反应。众所周知, pfp 基团形成低能量表面, 因此聚 (ppa) 刷子不能在水中很好地溶解28。对于 ip 应用, 需要将抗体固定在聚 (pfpa) 刷子上, 这种交换反应是在水缓冲液中进行的, 以保持抗体的活性。正如我们在上一份出版物中所报道的, 用氨基功能化的 peg 等亲水分子部分取代 pfp 单元可以提高表面亲水性, 从而提高抗体固定效率 18.在本研究中, 还制备了部分 peg 取代聚 (pfpa), 然后使用相同的 aptes 链接分子接枝到 sio2 表面。总体而言,图 1所示的方法允许制备具有不同程度 peg 取代的聚 (pfpa) 接枝表面。这些具有可调谐表面特性的聚合物刷子为抗体固定化和随后的 ip 应用提供了一个理想的平台。
珠子制备过程由 dls 和 xps 进行监控。图 2总结了 dmso 中各种功能化 sio2 磁珠的 dls结果。裸sio2微珠的流体动力直径为666纳米, 与制造商报告的珠子尺寸 (0.676 微米;sd = 0.03 微米)。经过 aptes 处理后, 珠径增加到740纳米;而聚 (pfpa) 处理后, 珠子直径进一步增加到1889纳米。必须指出的是, 聚 (pfpa) 接枝珠的多分散性指数 (pdi) 相当大 (pdi = 0.76), 这表明样品质量差, 含有大量的集料。虽然 dls 曲线只显示一个纳米大小的峰值, 但悬架中可能存在少量的聚集体。xps 还对功能化的 sio2 微珠进行了检测, 以确定表面成分 (图 3)。在 aptes 处理后, 检测到与 aptes 上的胺组相关的 n 1s 峰值。而且, 经过聚 (ppa) 处理后, 检测到与聚合物上的 pfp 单元相关的 f 1s 峰值。这些数据共同显示了 sio2 表面的成功功能化 , 首先是 aptes, 然后是聚 (pfpa)。
为了证明聚 (ppa) 接枝珠可以通过 ip 用于蛋白质的富集, 我们使用了1% 的 peg-e-聚 (pfpa) 接枝微珠, 并在没有抗体的情况下孵育它们, 一种非特异性兔 igg 抗体混合物, 或抗 pkr 抗体。从细胞中提取含有目标 pkr 的细胞裂解物, 然后利用三种类型的珠子通过 ip 进行 pkr 富集。为了确定 ip 效率, 通过西方印迹对两种不同抗体的洗脱蛋白样本进行了分析。采用抗 pkr 抗体对 pkr 的回收率进行了可视化。而且, 抗 gapdh (甘油醛-3-磷酸脱氢酶) 抗体被用作阴性对照, 因为 gapdh 是一种与 pkr 不相互作用的丰富蛋白质。如图 4所示, 没有抗体或非特异性抗体混合物固定化的珠子不会导致 pkr 恢复。相反, 用抗 pkr 抗体培养的珠子可以成功地丰富 pkr, 这表现在存在强的 pkr 带和没有 gapdh 带。这些结果表明, 聚乙二醇-取代聚多体 (pfpa) 刷子确实可以用抗体功能化, 并用于目标蛋白的选择性浓缩。请注意, 在比较不同珠子系统的蛋白质回收效率时, 应同时进行 ip 实验以及随后的西方印迹分析。由于进行这些实验的固有差异, 在单独试验中获得的数据不应直接比较。
正如前面所报道的, 聚 (pfpa) 刷的表面亲水性在 ip 效率18中起着关键作用。图 5显示了西方使用 0% peg 取代聚 (pfpa) 接枝珠和 1% peg 取代聚多聚 (pfpa) 接枝微粒子回收的 ip 蛋白样品的吸墨图数据。在这两种情况下, 珠子都被抗 pkr 抗体固定。虽然使用 0% ppeg 取代聚 (pfpa) 会导致 pkr 回收率低, 但 1% peg-e-取代聚 (pfpa) 的选择性富集表明, 目标 pkr 比非目标 gapdh 有选择性富集, 表明 pkr 有显著改善。与我们以前的出版物18一致, peg 处理提高了聚 (ppa) 刷子的表面亲水性, 使更多的 pfp 单元能够用于抗体固定, 从而提高了 ip 效率。请注意, 本研究中报告的 peg 替代百分比不能与我们以前的研究中报告的使用 si-raft 合成聚谱 (pfpa) 刷的百分比直接比较。这两种情况采用了非常不同的聚合物刷制备方法, 因此在 peg 负载相等的情况下, 可以使用的 pfp 单元数量预计会有很大的不同。然而, 这两项研究的观察结果在定性上确实一致, 都指出表面亲水性是实现高 ip 效率的关键控制参数。
虽然表面亲水性会影响聚 (pfpa) 刷子的抗体附着量, 但由于非特定富集, 它对 ip 背景也有显著影响。在一个典型的 ip 实验中, 许多洗涤步骤被用来去除未结合的蛋白质。当珠子非常疏水, 如那些与 0% peg 替代, 他们往往形成大的聚集体, 很难打破。在这种情况下, 非特异性蛋白质可以被困在集合结构内, 洗涤不能充分去除它们, 从而导致背景的增加。因此, 在执行 ip 时, 优化珠子表面性能是非常重要的, 应注意确保珠子的合理分散。
总体而言, 我们展示了一个简单的两步工艺来制备聚 (pfpa) 接枝的 sio 2 珠子, 并表明, 珠子的表面亲水性可以微调与 pfp 单位的部分替代氨基-peg。这些聚合物刷子通过 ip 成功地用于目标蛋白的浓缩, 成为传统的基于蛋白质 a 的 ip 技术的替代品。我们期望聚 (pfpa) 刷在许多其他需要生物分子固定的领域中得到应用。
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
作者没有什么可透露的。
Acknowledgments
这项工作得到了国防发展机构的支持 (第1号批准。ud170039 id)。
Materials
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
References
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Tags
化学 第141期 聚 (丙烯酸五氟苯基酯) 3-氨基丙基三乙氧基硅烷 活性聚合物刷 聚合后功能化 抗体固定 免疫沉淀Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 04/30/2019.
Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:
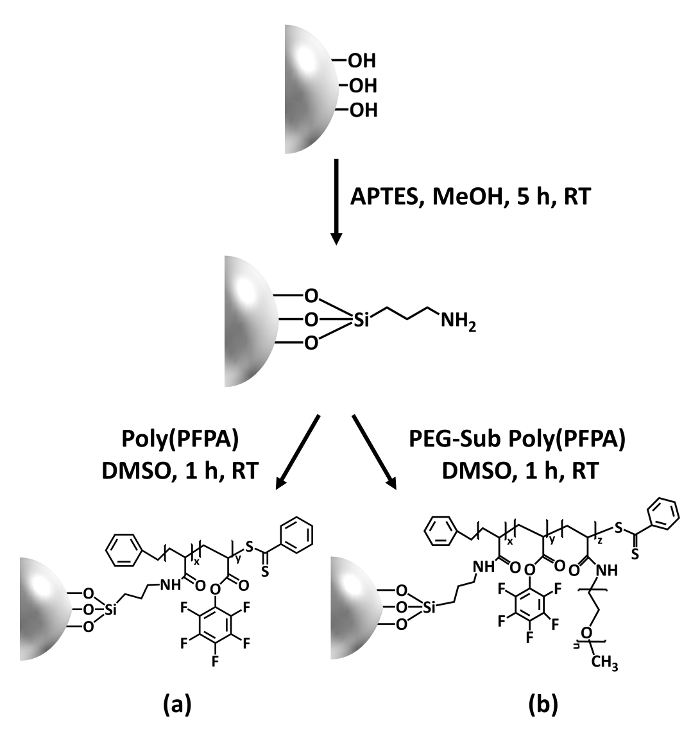
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
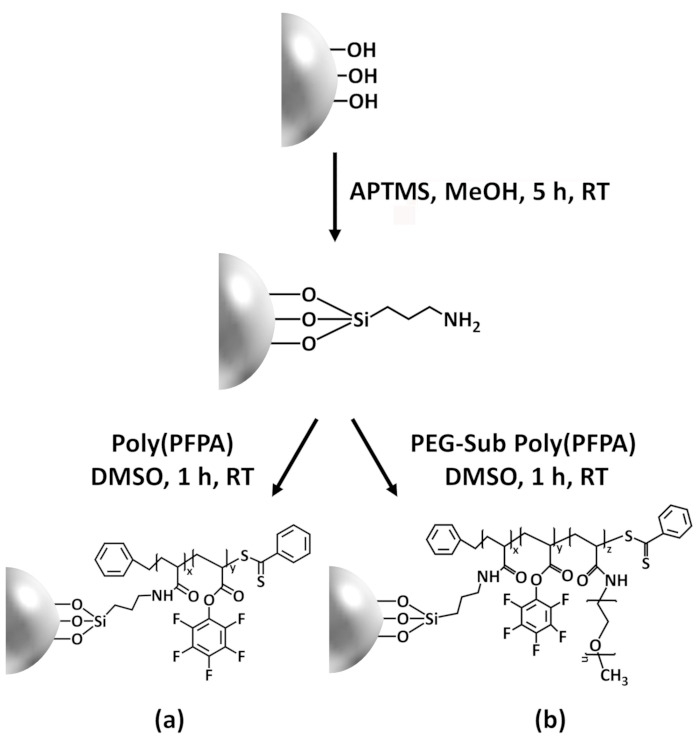
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
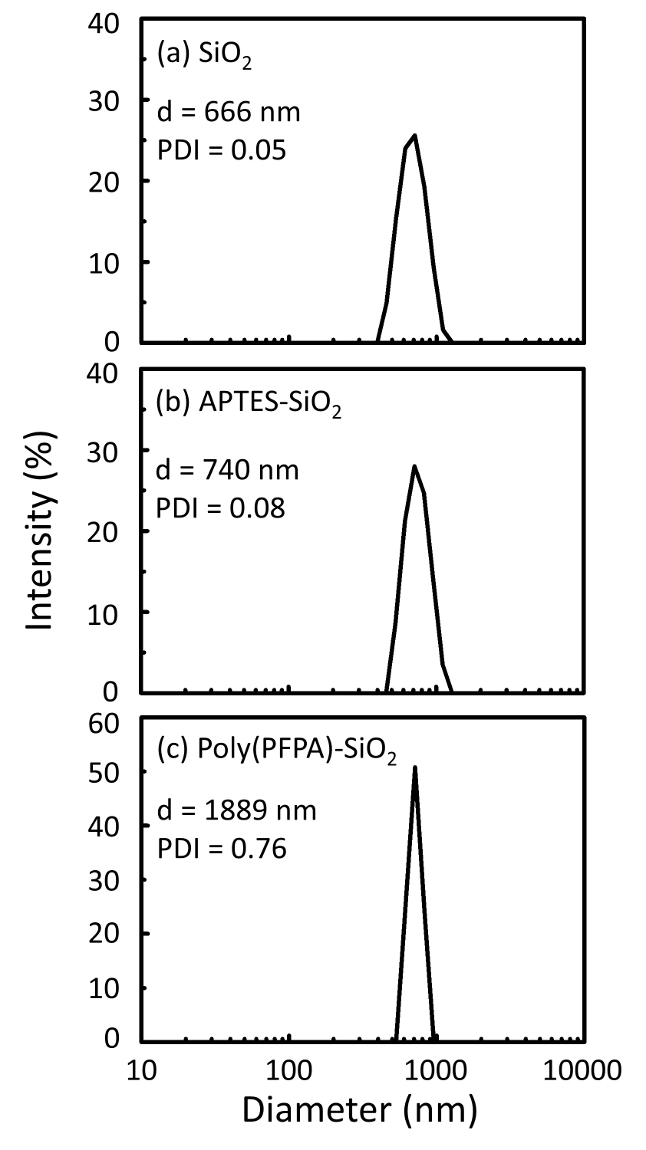
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
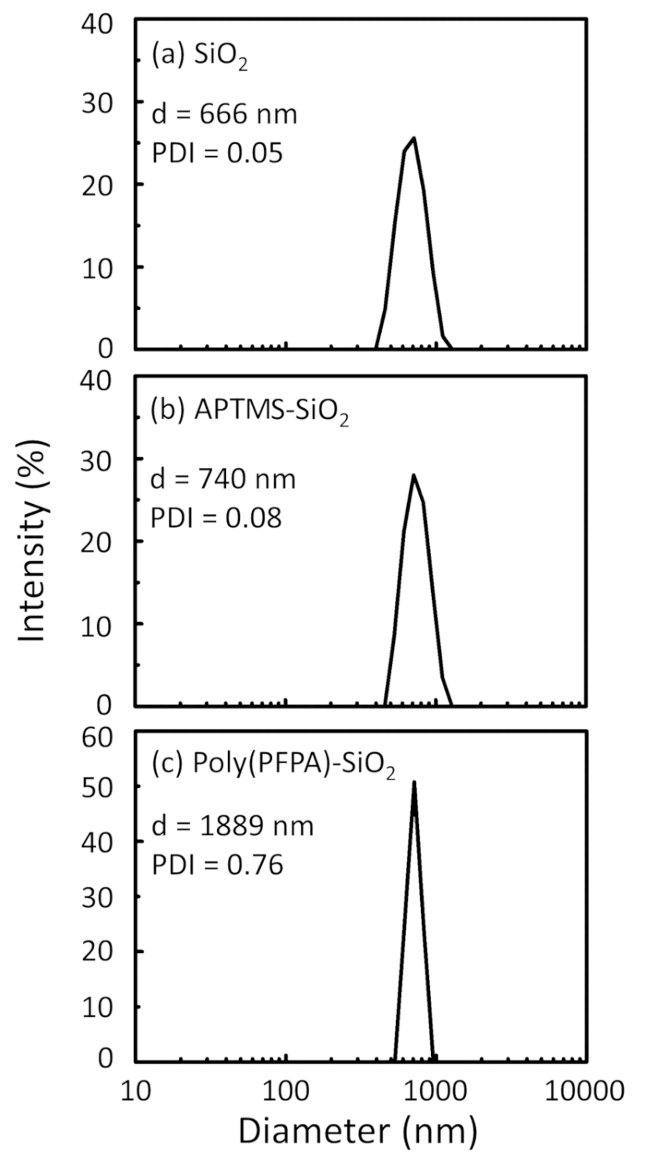
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
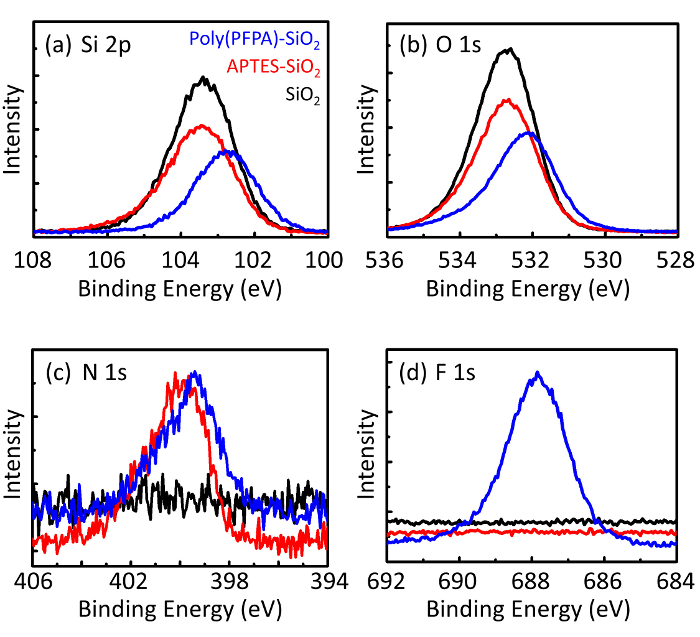
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
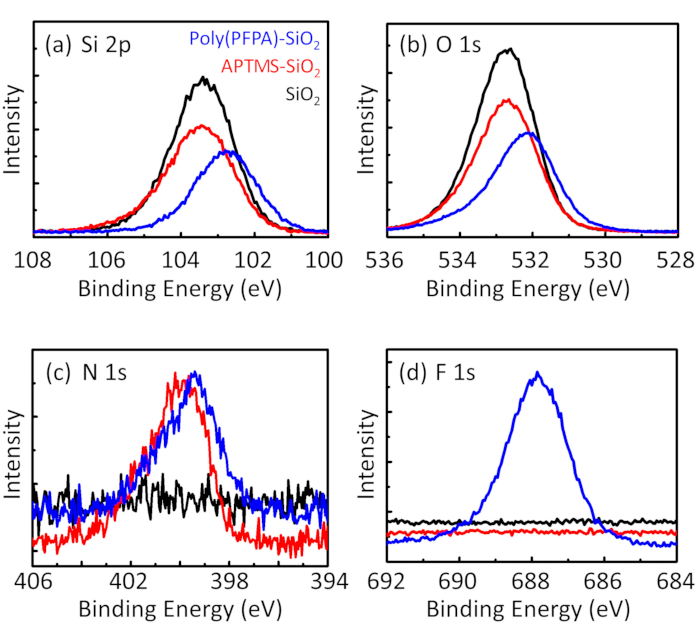
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).



