- 00:03Concepts
- 02:33Preparation of Materials and Dissection
- 03:39Immune Cell Isolation
- 05:22Magnetic Labeling of Immune Cells
- 06:59Magnetic Separation of CD3-Positive Cells
- 07:48Evaluation of Target-Cell Enrichment by Flow Cytometry
- 09:00Data Analysis
Magnetic Activated Cell Sorting (MACS): isolamento dei linfociti T timici
English
Share
Overview
Fonte: Meunier Sylvain1,2,3, Perchet Thibaut1,2,3, Sophie Novault4, Rachel Golub1,2,3
1 Unità di Linfopoiesi, Dipartimento di Immunologia, Istituto Pasteur, Parigi, Francia
2 INSERM U1223, Parigi, Francia
3 Université Paris Diderot, Sorbonne Paris Cité, Cellule Pasteur, Parigi, Francia
4 Platfrom, Citometria a flusso e biomarcatori UtechS, Center for Translational Science, Pasteur Institute, Parigi, Francia
La difesa contro gli agenti patogeni dipende dalla sorveglianza da parte del sistema immunitario. Questo sistema è complesso e comprende molti tipi di cellule, ognuna con funzioni specifiche. Questa complessa composizione consente risposte immunitarie a una grande varietà di agenti patogeni e lesioni. L’immunità adattativa consente risposte specifiche contro agenti patogeni specifici. La maggior parte delle cellule responsabili di questo tipo di immunità sono i linfociti (cellule B e cellule T). Di solito, le cellule B rispondono alle infezioni extracellulari (come le infezioni batteriche) e le cellule T rispondono alle infezioni intracellulari (come le infezioni virali). I diversi tipi di cellule nelle popolazioni di linfociti possono essere caratterizzati dalla combinazione di proteine di superficie cellulare che esprimono e/o da un pannello di citochine secrete.
La selezione magnetica consente l’arricchimento di popolazioni cellulari mirate utilizzando proprietà magnetiche ed espressione di una o più proteine di superficie cellulare (1, 2). Questa tecnica consiste di tre passaggi. In primo luogo, le cellule vengono incubate con perle magnetiche che sono accoppiate con uno o più anticorpi monoclonali specifici. Le cellule che esprimono proteine di superficie che si legano a questi anticorpi si attaccano alle perle magnetiche. Quindi, le popolazioni cellulari mirate vengono catturate con un magnete. Per finire, le cellule bersaglio vengono eluite dal magnete. Alla fine, si ottengono due prodotti di selezione, uno contenente cellule non etichettate e il secondo contenente le cellule bersaglio accoppiate con le perli magnetiche. Le colonne possono essere utilizzate per migliorare l’efficienza dello smistamento magnetico. Nella colonna, un elemento non magnetico allunga il percorso della cella attraverso la colonna. Quindi, il flusso cellulare viene rallentato, facilitando la cattura cellulare da parte del magnete.
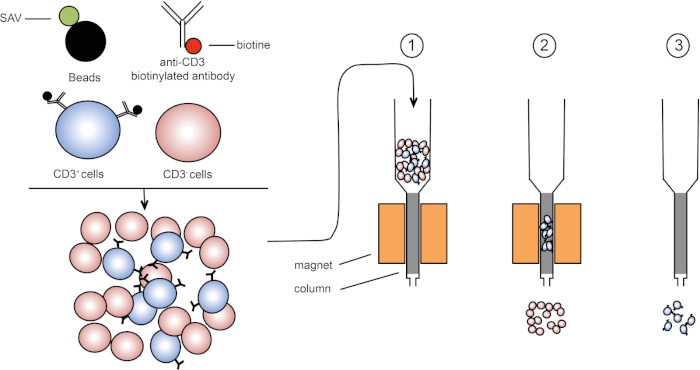
Figura 1: Rappresentazione schematica della separazione magnetica. I leucociti timici sono colorati con anticorpi biotinilati anti-CD3. Dopo il lavaggio, le perle accoppiate alla streptavitina (SAV) fissano specificamente la biotina sugli anticorpi anti-CD3. (1) Le celle vengono trasferite in una colonna. (2) Il magnete non trattiene le celle non etichettate, mentre le celle CD3-positive rimangono nella colonna. Infine, la colonna viene separata dal magnete e (3) le cellule CD3-positive vengono eluite nel mezzo. Fare clic qui per visualizzare una versione più grande di questa figura.
Esistono due tipi di smistamento magnetico (3). Nella selezione positiva, le cellule di interesse vengono catturate con le pere magnetiche. Nella selezione negativa, le cellule indesiderate vengono rimosse catturando con le pere magnetiche che trasportano gli anticorpi appropriati. Questa tecnica MACS consente un buon arricchimento delle cellule bersaglio e migliora la percentuale di cellule recuperate dall’1-20% al 60-98% in un organo. Dopo lo smistamento, è necessario verificare la purezza cellulare e lo smistamento con metodi diversi (ad esempio citometria a flusso). La tecnica MACS è ideale per arricchire una popolazione target per altri esperimenti come la coltura cellulare o l’analisi del ciclo cellulare.
In questo esercizio di laboratorio, dimostriamo come isolare i leucociti timici e successivamente arricchire le cellule timiche CD3-positive dal mix usando la tecnica di selezione delle cellule magnetiche.
Procedure
Results
In this protocol, CD3-positive cells were enriched from thymic leukocytes using magnetic cell sorting (Figure 1). Before magnetic cell enrichment CD3-positive cells represented 53.6% of the total thymic cells (Figure 2, top panels). After magnetic cell enrichment the percentage of CD3-positive cells increased to 95% (Figure 2, bottom panels). Thus, MACS is a simple, fast and efficient cell enrichment technique to enrich desired cell populations from a cell suspension mixture.

Figure 2: Gating strategy and purity test sorting. Cells are first gated based on their morphology (left: FSC-A, SSC-A), and then cells are plotted against CD3 (right: CD3, SSC-A). Top panel represents thymus cell suspension before cell enrichment. Bottom panel represents thymus cell suspension after magnetic cell sorting. Please click here to view a larger version of this figure.
Applications and Summary
Magnetic separation technology is a common method to easily and rapidly sort a target cell population. Using T cells specific antibodies and magnetic beads we enriched T cells frequency in our sample. The purity rate at the end of the experiment depends of the percentage of target cells in the initial cell suspension. Cells obtained after magnetic cell sorting can be used for various purposes such as- cell transfer or cell cycle analysis. Another sorting method, using flow cytometry, can be used to enrich cells. This technique yields have a very high purity rate after cell sorting however it requires more steps and takes more time.
References
- Owen, C. S. and Sykes, N. L. Magnetic labeling and cell sorting. Journal of Immunological Methods. 73 (1), 41-48 (1984).
- Miltenyi, S., Müller, W., Weichel, W. and Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry. 11 (2), 231-238 (1990).
- Plouffe, B. D., Murthy, S. K. and Lewis, L. H. Fundamentals and application of magnetic particles in cell isolation and enrichment: a review. Reports on Progress in Physics. 78 (1), (2014).
Transcript
Magnetic-activated cell sorting, or MACS, is a technique that allows researchers to separate cells based on specific epitopes expressed on their surfaces.
The process typically begins with extraction of an organ or tissue, such as the thymus. Then, the cells are mechanically separated, usually by crushing, until the tissue is dissociated into single cells. Unwanted cells can be removed at this stage via the addition of chemicals. For example, ammonium-chloride-potassium, or ACK buffer, can be used to lyse unwanted erythrocytes.
Next, an antibody conjugated to a molecule called biotin is added to the suspension, and these complexes bind to the epitopes of the surface of the target cells. Biotin has a high affinity for another molecule called streptavidin. In the next step, streptavidin molecules fused to magnetic beads are added to the antibody labeled cells. When the biotin and streptavidin come into contact, they tightly bind. The result is that the cells of interest are coated with magnetic beads. This complex is sometimes referred to as a sandwich. In this case, CD3 on the cell membrane on the bottom, then anti-CD3 conjugated to biotin, and finally, streptavidin conjugated to magnetic beads.
These labeled cells can now be placed into a column containing a matrix which, assisted by gravity, allows the cells to pass slowly by a magnet. As they do so, the magnetic bead-labeled cells will stick to the edge of the tube nearest the magnet, while the non-labeled cells will continue on into a collection tube below. Next, the labeled cells can be removed from the column by simply removing the magnet, adding an eluent solution, and applying gentle pressure with a plunger to flush them out of the column and into a fresh collection tube. Ultimately, this process allows for 60 to 98% retrieval of the cells of interest.
In this procedure, we will isolate thymic leukocytes from a mouse and use MACS to sort out CD3-positive T-cells before confirming the efficiency of sorting using FACS.
To begin, put on any appropriate protective equipment including a lab coat and gloves. Next, wash a pair of dissecting scissors and forceps with 70% ethanol and dry them with a clean paper towel. Then prepare 200 milliliters of HBSS 2% fetal calf serum, or FCS, by mixing four milliliters of FCS with 196 milliliters of HBSS.
Pin a euthanized mouse in a supine position on a dissection plate. Using scissors and forceps, perform a longitudinal laparotomy to access the chest cavity. First, remove the heart to gain access to the thymus, which is located above the heart. Then identify the thymus, which is composed of two white lobes. Using forceps, carefully detach the thymus and place it on a Petri dish with five milliliters of HBSS 2% FCS.
To isolate the immune cells, first place the thymus on a 40 micrometer cell strainer in the Petri dish. Crush the tissue with a plunger to dissociate it into the dish. After this, rinse the plunger and strainer with HBSS 2% FCS to recover any adhered cells. Then, pipette the dissociated thymus cells and fluid from the Petri dish into a 15 milliliter centrifuge tube. Wash the Petri dish with five milliliters of HBSS 2% FCS and transfer this wash solution to the 15 milliliter centrifuge tube also.
Next, centrifuge the tube at 370 times g for seven minutes at 20 degrees Celsius. Discard the supernatant and resuspend the pellet in two milliliters of ACK lysing buffer to lyse the erythrocytes. Incubate for two minutes at room temperature on the bench top. Then, bring the volume to 14 milliliters with HBSS 2% FCS. Centrifuge the tube at 370 times g for seven minutes at 20 degrees Celsius. Then, discard the supernatant and resuspend the cells in five milliliters of HBSS 2% FCS.
Estimate the cell concentration using a Malassez slide as shown in the protocol for FACS isolation of B lymphocytes and adjust the cell concentration to 10 to the seventh cells per milliliter with HBSS 2% FCS.
Transfer 500 microliters of cell solution into two FACS tubes. Label one tube non-enriched T-cells and the other tube enriched T-cells, which will be separated using magnetic labeling.
Centrifuge the enriched T-cells tube at 370 times g for three minutes at 20 degrees Celsius. Discard the supernatant and resuspend the pellet in 250 microliters of biotin coupled anti CD3 antibody diluted one in 400 in HBSS 2% FCS. Incubate the cells for 20 minutes on ice and in the dark. Add three milliliters of HBSS 2% FCS to the tubes and centrifuge them again at 370 times g for three minutes at 20 degrees Celsius. Discard the supernatant and resuspend the pellet in 250 microliters of streptavidin-coupled beads diluted one in five in HBSS 2% FCS. Incubate the mixture of cells and beads for 20 minutes on ice. Next, add three milliliters of HBSS 2% FCS to the tube, pipette up and down to mix, and centrifuge again at 370 times g for three minutes at 20 degrees Celsius. Resuspend the pellet in two milliliters of HBSS 2% FCS.
Place the column on the magnet and add three milliliters of HBSS 2% FCS to humidify the system. Then, pipette the stained cells into the column. After the cell suspension passes through the column, wash the column three times with three milliliters of HBSS 2% FCS. Next, remove the column from the magnet and place it in a 15 milliliter tube. To elute the target cells, add five milliliters of HBSS 2% FCS to the column and flush the column with a plunger. Repeat this step with another five milliliters of HBSS 2% FCS.
To evaluate the effectiveness of target cell isolation, first transfer 500 microliters of eluted cell suspension to a FACS tube and label it enriched T-cells. Then, centrifuge both the enriched and non-enriched tubes at 370 times g for seven minutes at 20 degrees Celsius. Discard the supernatant, then add 100 microliters of fluorescent antibody diluted one in 200 in HBSS 2% FCS to both tubes. Incubate the cells for 20 minutes on ice and in the dark. Next, add three milliliters of HBSS 2% FCS to the tubes and centrifuge them at 370 times g for three minutes at 20 degrees Celsius. Discard the supernatant, then resuspend the pellets in 250 microliters of HBSS 2% FCS. Now, evaluate the CD3-positive cell enrichment rate using flow cytometry as shown in the FACS protocol.
Now, we will determine the frequency of CD3-positive lymphocytes among all thymocytes that were isolated from the mouse thymus. To start, double click on the FlowJo icon and drag the files for each tube in the all sample window. Then, double click on the enriched T-cells file to display the cells recorded from that sample on a dot plot that displays forward scatter, FSCA, on the x-axis, and side scatter, SSCA, on the y-axis.
Click on polygon to circle the lymphocyte populations. Next, double click on the circled population to create a new window. Select FSC-W on the y-axis, and FSC-A on the x-axis and circle the FSA-W negative cells. In the sub population identification window, name your cell population Single Cells. Next, click on OK on the sub population identification window, then double click on the circled population to create a new window. Select CD3 on the y-axis, and circle the CD3-positive cells. In the sub population identification window, name your cell population T-cells. Repeat with the non-enriched T-cells file. To visualize your cell population, click Layout Editor and drag the T-cell population from enriched T-cells and non-enriched T-cells files into the tab.
Dot plots representing CD3-positive lymphocytes will appear. CD3-positive cells should only appear in the population of interest in the CD3-positive enriched tube. To evaluate the enrichment of CD3-positive lymphocytes in the sorted cells, click on Table Editor and then drag the T-cells population from enriched T-cells and non-enriched T-cells files into the table. On the statistic menu, select Frequency of Lymphocyte Cells to check the percentage of CD3-positive cells in all lymphocytes. Then, click on Create Table. Parameter values will appear in a new table. For the enriched T-cells, the frequency of CD3-positive cells should be around 80% or above.
