- 00:01Concepts
- 04:08Preparation of Plates and Bacteria
- 06:04Determining MIC Using E-Test
- 07:01Synergy Testing: Cross Approach
- 07:47Synergy Testing: Non-Cross Approach
- 08:56MIC Determination Using Broth Dilution
- 10:33Data Analysis: Broth Microdilution
- 11:10Data Analysis and Results: Synergy Testing
抗生素敏感性测试:Epsilo计测试,以确定两种抗生素的MIC值,并评估抗生素协同效应
English
Share
Overview
资料来源:安娜·布吕克伯格1, 罗尔夫·卢德1
1临床科学系 隆德,感染医学系,生物医学中心,隆德大学,221 00 隆德瑞典
了解抗生素和细菌之间的相互作用对于了解微生物如何进化抗生素耐药性非常重要。1928年,亚历山大·弗莱明发现了青霉素,一种通过干扰细胞壁再生来发挥抗菌作用的抗生素(1)。随后发现了其他具有不同作用机制的抗生素,包括抑制细菌DNA复制和蛋白质转化的药物;然而,近年来没有开发任何新的抗生素。对现行抗生素的耐药性一直在增加,导致无法有效治疗的严重传染病(2)。在这里,我们描述了几种评估细菌群抗生素耐药性的方法。这些方法中的每一个都有效,无论所使用的抗生素的作用机制如何,因为细菌死亡是测量的结果。抗生素耐药性不仅通过医院环境迅速传播,而且在全社会迅速传播。为了研究这种抗性手段,已经开发出了不同的方法,包括Epsilo计测试(E测试)和肉汤稀释试验(3)。
电子测试是一种公认的方法,是一种具有成本效益的工具,可量化最小抑制浓度 (MIC) 数据,这是抑制微生物可见生长的抗菌素的最低浓度。根据所使用的细菌菌株和抗生素,MIC 值在子 μg/mL 到 >1000 μg/mL (4) 之间可能有所不同。E-test 使用包含预定义的抗生素梯度的塑料条进行,该塑料条以 μg/mL 为单位与 MIC 读数表一起印记。当应用于接种琼脂板时,该条带直接在琼脂基质上传输。孵育后,随着细菌生长的预防,沿带可见对称的椭圆抑制区。MIC 由抑制区域定义,该区域是椭圆与条带相交的端点。另一种确定MIC的常见方法是微溴稀释法。微溴稀释含有不同浓度的抗菌剂添加到含有接种细菌的汤培养基中。孵育后,MIC被定义为阻止可见生长的最低浓度抗生素(5)。它也是一种定量方法,可应用于多种细菌。该方法的缺点包括制备试剂浓度时可能存在错误,以及实验所需的大量试剂。从临床和研究的角度衡量抗生素耐药性是必须的,下面将讨论并展示这些研究耐药性的体外方法。
可以应用特定细菌的耐药性概况,以优化抗生素治疗,以确定患者是否会受益于联合治疗与单一治疗。对于一次使用多个抗生素,必须了解它们之间的相互作用,以及它们是否具有添加剂、协同或对抗作用。当抗生素的关节效应等于以等剂量给予的单个抗生素的效力时,可以看到添加剂效应。另一方面,当抗生素的联合作用比单独给予药物更有效时,抗生素之间的协同作用就存在(6)。应用抗菌治疗组合,避免抗菌素耐药性的发生,从而增强单个抗生素治疗的效果(7)。对抗知识对于防止不必要地使用抗菌素组合也同样重要。电子测试方法提供了简单和多种方法,以确定不同抗菌剂之间可能的协同作用和对抗。为了面对抗生素耐药病原体的扩散,了解某些抗生素的可能协同和对抗机制对于临床疗效和对抗多药耐药性非常重要。
使用电子测试确定协同效应可以分为两大类:交叉测试和非交叉测试。虽然这两种协同测试都依赖于以前对各个 MIC 值的了解,但两种方法在方法和概念方法上略有不同。在非交叉协同测试中,被测试的对中的第一个抗生素被放置在接种细菌的琼脂板上。允许从第一条中注入板中的抗生素(例如,在 1 小时后),去除该条,并将包含第二种抗生素的新条放在与第一个抗生素完全相同的位置,确保将两个单独的 MIC 值放在每个 ot 的顶部她。然后,可以按上述方式分析产生的抑制区,并根据公式 1 计算协同效应。
公式 1 – 分数抑制浓度 (FIC)
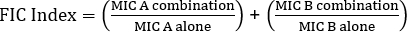
值 >0.5 显示了协同效应。
虽然用易于分析的板来奖励考官,但由于条带的变化,以及每个实验需要使用两个板,这种方法有些费力和费时。相反,通常采用交叉测试。而不是添加两个不同的电子测试条随后彼此的顶部(删除第一个后),两者同时放置,但以十字(90° 角)的形式,与两个先前确定的MIC值形成90°角。通过这种方法,每个协同测试只需要一个板,而且工作更少,尽管分析起来稍微困难一些,但还是成为首选。组合抗生素方法中新的MIC值可以可视化为经过修饰的抑制区,之后可以通过公式1确定协同效应。
角)的形式,与两个先前确定的MIC值形成90°角。通过这种方法,每个协同测试只需要一个板,而且工作更少,尽管分析起来稍微困难一些,但还是成为首选。组合抗生素方法中新的MIC值可以可视化为经过修饰的抑制区,之后可以通过公式1确定协同效应。
微broth方法通常具有更高的灵活性(例如,在电子测试条限制之外选择特定浓度的抗生素的能力),而不是使用琼脂板方法,通常可以优先使用。此外,微broth测试建议更敏感,因为它们在液体溶液中均匀分布抗生素,而不是取决于固体相(琼脂板)内的解散。96孔微孔中的孔将接种一组细菌(106 cfu/mL:细菌浓度可通过OD600 nm测量、浊度标准或从10倍细菌系列稀释中传播电镀样品来估计),以及不同稀释液中的抗生素将被添加到井中。同样,对于电子测试条,MIC被确定为与抗生素浓度最低抑制细菌可见生长的交叉点(井/点)。
实验目标
- 以下项目描述了通过两种不同的方法确定青霉素G和链球菌组G的基母菌素的MIC值的策略,即电子测试和微溴稀释。在电子测试中,用G链球菌组接种的Mueller-Hinton琼脂板与青霉素G和/或文母素的梯度条结合使用;而MH-broth与50%溶化马血和20mg/mLβ-NAD一起使用可溶性抗生素与链球菌组G在微broth方法。
材料
- 血琼脂板上的细菌菌落,在4°C中储存<7天
- 血琼脂板
- 0.5 麦克法兰标准
- 1% 巴克莱2
- 1% H2SO4
- 盐管 (2 mL)
- 棉尖施用器
- 穆勒-欣顿琼脂板(MHA板)
- 含有50%裂裂马血和20mg/mL +-NAD(MH-F)的MH肉汤
- 电子测试青霉素/金霉素(或感兴趣的抗生素)(BioMerieux,马西埃托莱,法国,瑞典)
- 抗生素青霉素/青霉素(或感兴趣的抗生素(粉末/溶液))
注意:用于细菌生长的特定介质可能因不同物种而异。
Procedure
Results
MIC values in E-test
Individual MIC values were identified in Figure 1 as 0.094 μg/mL for penicillin G and 8 μg/mLfor gentamicin. For synergy tests, both demonstrated an MIC value for penicillin G of 0.064 μg/mL (Figures 2, 3), while gentamicin had an MIC 4 μg/mL for cross and non-cross tests. Note a slight discrepancy between the cross and non-cross tests may occur due to the different incubation times of the strips in the two settings.
Calculation of synergy
The equation for FIC is:
 = 1.18 >0.5 (no synergy)
= 1.18 >0.5 (no synergy)
MIC determination in broth
Cloudiness of the wells indicated bacterial growth, and thus no inhibition occurred. The first clear well with penicillin G (Figure 4) contained 0.12 μg/mL penicillin G, and hence this was the MIC value. For gentamicin the first clear well was present at 8 μg/mL gentamicin. The penicillin G value was slightly higher than when using an E-test, due to the higher resolution of the strip (e.g. based on a 1.5x factor serial dilution, not a 2x factor).
Inoculum size
To determine the inoculum size, an approach as outlined in Figure 5 and 6 was used. Colonies were counted in the D-row (1000x dilution), adding up to 7, 8, and 8 in the triplicate series with a mean value of 7.67 cfu. The number of colonies neeed to be multiplied with the dilution factor (e.g. 1000x), as well as with 100 to obtain cfu/mL, giving an inoculum size of approximately 8 x 105, well within the targeted inoculum size of 105-6 cfu/mL.
Applications and Summary
Antibiotic resistance is a worldwide health problem. In order to determine resistance mechanisms of microbes, methods testing for synergy and antagonism with different antibiotics is crucial. The E-test method is rapid, easy to replicate, and can be used to investigate any synergistic potential of combination therapies. The broth dilution method can also be assessed to predict bactericidal activity. In order to investigate the resistance mechanisms of different microbes, knowledge of synergistic and antagonistic antibiotic interactions is crucial. Combining antibiotics may be a strategy to increase treatment efficacy and face antibiotic resistance. In the tests performed here, we were able to determine the MIC values of penicillin G and gentamicin for group G Streptococcus. We also demonstrated that the two antibiotics do not display synergistic effects, thus would not be a preferred treatment option for such infections.
References
- Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): Discoverer of penicillin. Singapore Medical Journal. 56 (7):366-7. (2015)
- Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Frontiers in Microbiology. 1:134. (2010)
- Pankey GA, Ashcraft DS, Dornelles A. Comparison of 3 E-test (®) methods and time-kill assay for determination of antimicrobial synergy against carbapenemase-producing Klebsiella species. Diagnostic Microbiology and Infectious Disease. 77 (3):220-6. (2013)
- EUCAST: European Committee On Antimicrobial Susceptibility Testing (www.eucast.org).
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 3 (2):163-75. (2008)
- Doern CD, When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. Journal of Clinical Microbiology. 52 (12):4124-28. (2014)
- Worthington RJ, Melander C. Combination approaches to combat multi-drug resistant bacteria. Trends in Biotechnology. 31 (3):177-84. (2013)
Transcript
Antibiotic susceptibility is defined as the sensitivity of a bacteria to antibiotics and can be measured using a broth dilution test or an Epsilometer test, also called an E-test.
In the broth dilution method, a standardized number of bacteria are added to a growth media containing serial antibiotic dilutions. If susceptible, the bacteria cannot grow at the higher antibiotic concentrations but continue to multiply at the lower antibiotic concentrations, causing media to turn turbid. The lowest antibiotic concentration at which the bacteria can no longer survive or multiply is referred to as the minimum inhibitory concentration, or MIC, value of the antibiotic for the given bacteria.
In an E-test, a plastic strip impregnated with a predefined gradient of antibiotic is applied over a freshly spread lawn of bacteria on a Mueller-Hinton agar, or MH-A, Petri plate. The antibiotic diffuses out into the agar media, where it is taken up by the bacteria. If susceptible, the bacteria cannot multiply and will die off, forming a clear zone around the E-strip, which is referred to as the growth inhibition zone. At the point where the growth intersects with the E-strip, the corresponding value on the scale gives the MIC value of the antibiotic.
Often antibiotics are used in combination to prevent the emergence of antibiotic resistant strains of bacteria. This often results in a synergistic, rather than additive, effect. Synergistic means that the combined effect of the two antibiotics is greater than the sum of their individual activities. However, the effect is considered significant only when the MIC value of the antibiotic combination decreases by at least two-fold. This criterion is evaluated by calculating the fractional inhibitory concentration, or FIC, index. By summing the ratio of the MIC of each antibiotic in combination with the MIC of each antibiotic individually, an FIC index less than 0.5 indicates synergy.
Antibiotic synergy can be measured using two E-test based methods: a non-cross test or a cross test. In a non-cross test, first, the E-strips for two different antibiotics with predetermined MIC values are applied to two separate plates. After the antibiotics have diffused into the medium, the original E-strips are removed and the E-strips for the alternate antibiotics are placed such that their MIC scales lay exactly over the MIC scales of the previous strips. In a cross test, which is a faster version of the non-cross test, the E-strips of the two antibiotics are placed together in a cross formation, such that the scales of their MIC marks form a 90 degree angle at the intersection. Following incubation in both techniques, the MIC value of each antibiotic in combination with the other antibiotic is read at the point where the growth inhibition zone intersects with the edge of the E-strip. Then, the FIC index is calculated.
This video will demonstrate how to determine the MIC value of a given antibiotic for a given bacteria using an E-test and a micro broth dilution test. You will also learn how to determine synergy between two antibiotics using a cross test and a non-cross test.
To begin, put on any appropriate personal protective equipment, including laboratory gloves and a lab coat. Next, sterilize the work space using 70% ethanol. Next, collect 15 milliliters of sterile Mueller-Hinton broth with 50% lysed horse blood and 20 milligrams per milliliter beta-nicotinamide. And five to eight Mueller-Hinton agar plates. Now, to prepare a McFarland turbidity standard number 0.5, measure out 9.95 milliliters of 1% sulfuric acid solution. Then, add 50 microliters of 1% barium chloride solution to the sulfuric acid solution. Vortex the solution well to obtain a turbid suspension. Cover the tube with aluminum foil and set it aside. Next, dispense one milliliter of saline solution into a 15 milliliter tube.
Use a sterile loop to scrape up a sample of the bacterial growth from your bacterial test plate, here, Streptococcus group G. Then, place the bacteria-laden loop into the saline solution, stir gently, and then vortex the tube well. Now, place the bacterial suspension and McFarland turbidity standards side by side and compare them for turbidity equivalence. Add either additional saline or bacterial colonies until the bacterial suspension’s turbidity matches that of the standard. Once the desired turbidity is obtained, dip a sterile cotton tip applicator into the bacterial suspension. To inoculate the MH-A plate, swab the entire surface of the plate gently with a zigzag motion. Next, label the bottom sides of the plates with the name of the bacteria and the date.
To begin, take out a penicillin G E-test strip, holding it by the edge with forceps. Gently place strip into the center of the freshly swabbed MH-A plate and replace the lid. In this example, a second antibiotic, gentamicin, is also tested. Thus, the strip placement process is repeated with the second plate and a gentamicin E-test strip. To determine the results of the E-test, collect the first plate that contains the penicillin G E-test strip. Now, determine the point where the inhibition zone intersects with the antibiotic strip. Read the corresponding numerical value on the scale. This value represents the MIC value of penicillin G. Determine the MIC value for gentamicin in the same manner.
To begin, inoculate an MH-A plate with Streptococcus group G strain bacteria. Label the bottom of the plate with the name of the bacteria, antibiotics to be used, and the date. Now, place an E-test strip for the antibiotic of interest in the center of the plate. Then, hold the second test strip at a 90 degree angle to the first strip and locate its MIC mark. Gently lay the second E-strip over the first at the point where the two MIC values intersect. Once the strips are placed, do not move them. Next, incubate the plates at 37 degrees celsius for 18 to 20 hours.
After inoculating two MH-A plates, with Streptococcus group G strain bacteria, place an E-test strip for one antibiotic on the surface of one plate. Then, place an E-test strip for the other antibiotic on the second plate as demonstrated. Using a plastic inoculation loop, mark the MIC value of each antibiotic on the surface of its respective plate. Next, cover the plates and incubate them at room temperature for one hour. After this, use forceps to remove the E strips. Next, collect one of the plates and an E-test strip for the other antibiotic. Hold the E-test strip over the imprint left by the first strip and locate the point where the MIC value on the E strip aligns with the marked line. Gently place the strip at this intersecting point. Repeat this process for the second plate and incubate both plates at 37 degrees celsius for 18 to 20 hours.
First, obtain a bacterial suspension with an established bacterial concentration and dilute the culture in MHF broth to achieve an OD600 of 0.003. Next, weigh out 16 milligrams of penicillin G and 128 milligrams of gentamicin. Transfer each weighed dry antibiotic into 215 milliliter conical tubes. Add 10 milliliters of distilled water to each of the conical tubes and mix well by vortexing. Label the tubes with the antibiotic name and concentration.
Performing the assay in triplicate, add 400 microliters of the working bacterial solution into the first wells of three rows of a 96-well microtiter plate. Next, add 200 microliters of the working bacterial solution in MHF broth to the wells of the three rows. Now, to generate a two-fold serial antibiotic dilution, first add four microliters of antibiotic stock to the first well, generating a 100 fold dilution. Sequentially, transfer 200 microliters of bacteria-antibiotic solution to each well, beginning from the first well through the second to last well in each row, ensure proper mixing by pipetting two to three times after every transfer. Discard the final 200 microliters of bacteria-antibiotic solution.
To determine the results of the broth micro dilution test for penicillin G, first locate the wells that exhibit no visible bacterial growth, indicated by a lack of turbidity. From these wells, identify the well with the lowest antibiotic concentration. This represents the MIC value of penicillin G for the tested bacteria. The MIC value of gentamicin can be determined using the same assay and technique.
To determine the results of the non-cross test, collect the first plate, which contains a penicillin G E strip. Then, determine the point where the growth inhibition zone intersects with the antibiotic strip. The corresponding value on the scale represents the MIC value for penicillin G in combination with gentamicin. In this example, the MIC value in combination is 0.064 micrograms per milliliter.
Now, collect the second plate, which contains the gentamicin E strip, and determine the MIC value in combination as previously demonstrated. To evaluate the effect of combination, first calculate the fractional inhibitory concentration or FIC for penicillin G by dividing the MIC in combination by the MIC of the antibiotic alone. Repeat this process for gentamicin. Then, calculate the FIC index using the equation shown here. A two-fold reduction in the MIC value in combination yields an FIC index value that is less than or equal to 0.5 and demonstrates synergy between penicillin G and gentamicin. In this case, the calculated FIC value is 1.18 which is greater than 0.5. Thus, the results do not demonstrate synergy between penicillin G and gentamicin against the Streptococcus group G strain.
To determine the results of the cross test, first determine the point where the growth inhibition zones intersect with their respective E strips. Read the numerical value on each E-test strip that corresponds to this intersection point. These values represent the MIC value in combination for penicillin G and gentamicin. Next, to evaluate the effect of the combination, calculate the FIC index using the equation shown here. In this example, the calculated FIC value is 1.18, which is greater than 0.5. This means that penicillin G and gentamicin do not act synergistically against the Streptococcus group G strain.
