Summary
Commonly used, highly accessible methods for examining cell migration and invasion in vitro are described. The first method is the cell wound closure assay that measures cell motility. The second method is the transwell migration and invasion assay that assesses the chemotactic and invasive capacity of cells.
Abstract
Migration is a key property of live cells and critical for normal development, immune response, and disease processes such as cancer metastasis and inflammation. Methods to examine cell migration are very useful and important for a wide range of biomedical research such as cancer biology, immunology, vascular biology, cell biology and developmental biology. Here we use tumor cell migration and invasion as an example and describe two related assays to illustrate the commonly used, easily accessible methods to measure these processes. The first method is the cell culture wound closure assay in which a scratch is generated on a confluent cell monolayer. The speed of wound closure and cell migration can be quantified by taking snapshot pictures with a regular inverted microscope at several time intervals. More detailed cell migratory behavior can be documented using the time-lapse microscopy system. The second method described in this paper is the transwell cell migration and invasion assay that measures the capacity of cell motility and invasiveness toward a chemo-attractant gradient. It is our goal to describe these methods in a highly accessible manner so that the procedures can be successfully performed in research laboratories even just with basic cell biology setup.
Introduction
Motility is an essential feature of live cells. Cell migration is involved in the conception of life, embryonic development, immune response, and many pathological processes such as cancer metastasis and inflammation1-9. Therefore, methods to study cell migratory behavior are very useful research tools for a wide range of disciplines in biomedical sciences, biology, bioengineering, and related fields.
The study of cell migration in cancer research is of particular interest as the main cause of death in cancer patients is related to metastatic progression. In order for cancer to spread and disseminate throughout the body, cancer cells must migrate and invade through extracellular matrix (ECM), intravasate into blood circulation, attach to a distant site, and finally extravasate to form distant foci1,10-12. Various biological methods may be employed to study these events in detail. The cell culture wound-closure and the transwell migration and invasion assays are widely used in the scientific community1,10. These tests can provide the necessary data that may allow for an understanding of how well a particular cell type can spontaneously migrate or respond to a chemo-attractant and directionally migrate toward it. Several migratory phenotypes have been described. Cells may migrate in a single cell form such as seen in mesenchymal or amoeboid-like movement or by multicellular movement labeled collective migration or cell streaming13. The method of movement used in motile cells can be readily observed using the cell culture wound closure assay.
Among the numerous ways to study cell migration, the cell wound closure assay is one of the simplest. This method is useful to determine the migration ability of whole cell masses. When taken a step further it can be used to observe individual cell’s morphological characteristics during migration14. Following the analysis of the wound closure many phenotypes may be revealed. Measuring the closed distance over time when comparing to a control may reveal specific migration changes or an impaired migratory phenotype that was unknown previously. Furthermore, single cell lamellipodium formation, tail retraction, and directional movement may give clues for what may be impaired or enhanced in the cells of interest14.
The transwell migration and invasion assays may be used to analyze the ability of single cells to directionally respond to various chemo-attractants whether they are chemokines, growth factors, lipids, or nucleotides4,5,8,15,16. It may also assess differential migratory ability due to the over-expression of a receptor1,14. These assays can also be used to identify and characterize the key regulators of cell migration such as the Rho family of small GTPases2. Following these short and easily accessible tests, the mode of cell migration and the ability of a cell to invade into a 3-D matrix may also be determined.
Protocol
1. Cell Culture Wound Closure Assay
- Detach cells from the tissue culture plate using 0.25% Trypsin-EDTA solution. Pellet cells in a 15 ml conical tube by centrifugation, aspirate the supernatant, and re-suspend cells in culture media. Plate the appropriate number of cells in a 6-well plate for 100% confluence in 24 hours.
Note: Tests may be needed to determine the time and number of cells to achieve 100% confluence due to the cell type and the size of the well being used (for example, 1 x 106 B16F10 melanoma cells are seeded in a well of a 6-well plate 24 hours prior to the wound generation). Alternatively, 12-well or 24-well plates may be used.
Note: If available, cells can be seeded into a Culture-Insert (from ibidi LLC) on a treated tissue culture plate making the wound pre-casted. A Culture-Insert can protect the surface of the tissue culture plate from damage by the pipette tip. If a Culture-Insert is used omit step 1.2. - In a sterile environment (typically a biosafety hood) use a 200 μl pipette tip to press firmly against the top of the tissue culture plate and swiftly make a vertical wound down through the cell monolayer. A different sized pipette tip may be used to make the wound size that is desired. Do not use excessive force against the tissue culture plate with the pipette tip as it may damage the surface. If the wound is pre-casted, this step may be omitted.
- Carefully aspirate the media and cell debris. Slowly add enough culture media against the well wall to cover the bottom of the well and avoid detaching additional cells. Following the generation and inspection of the wound an initial picture should be taken. Place the tissue culture plate in an incubator set at the appropriate temperature and CO2 concentration (typically 37 °C and 5% CO2).
- At several time points, e.g. every 3 hours, remove the plate from the incubator and place it under an inverted microscope to take a snapshot picture and to check for wound closure. If time-lapse microscopy is available, take a photo every X number of minutes (typically starting with every 5 minutes) for any time duration in a temperature and CO2 controlled chamber. Depending on the cell type, wound closure time may vary.
- To analyze the results of snapshot pictures, measure the distance of one side of the wound to the other using a scale bar. Analyze and clearly present wound closure over time using a scatter plot or bar graph, e.g. Figure 1.
- To analyze the time-lapse pictures, import the pictures into the ImageJ software(freely available at http://rsb.info.nih.gov/ij/) to make a video. To do this open ImageJ, click File, Import, and Image Sequence. Find the file with pictures; click the first photo in the file. Save video by selecting File, Save as, and AVI. Single migrating cells may be observed to characterize directional movement. Furthermore, morphological characteristics such as lamellipodia formation and trailing edge retraction may be studied as well (Figure 2 and supplementary video).
2. Transwell Cell Migration and Invasion Assay
- In a sterile environment (typically a biosafety hood) detach cells from the tissue culture plate using a non-enzymatic cell disassociation buffer or 0.25% Trypsin-EDTA solution, pellet cells by centrifugation, and aspirate the existing media leaving the pelleted cells. Re-suspend cells in serum free cell culture media containing 0.1% BSA (bovine serum albumin).
Note: Depending on the cell line being investigated 0.25% Trypsin-EDTA may affect cell migration and invasion due to the cleavage of various receptors on the cell surface17.
Note: The number of cells per ml depends on the size of the cells. Further tests may be needed to find a seeding density that provides the best results. Typically 1 x 106 cells/ml is a good starting point for most cell types when using the 24-well transwell insert. There is a range of transwell insert sizes and the number of cells added should be adjusted for accordingly. - Plate 100 μl of cell solution on top of the filter membrane in a transwell insert and incubate for 10 minutes at 37 °C and 5% CO2 to allow the cells to settle down.
Note: The particular pore size of the transwell membrane used is dependent on the individual size of the cells in the sample. For example, if the cells are too small they may pass through the pores without the need to facilitate migration or if they are too large they may not fit through the pores. There are several transwell inserts with pore sizes that range from 3 µm, 5 µm, and 8 µm for cell migration assays. The transwell inserts are commercially available from companies such as Corning.
Note: The transwell migration assay can be easily modified to perform the cell invasion assay. To do this, add extracellular matrix (ECM) materials on top of the transwell membrane and then add cells on top of the ECM. For example, Matrigel is thawed and liquefied on ice, and then 30-50 µl of Matrigel is added to a 24-well transwell insert and solidified in a 37 °C incubator for 15-30 minutes to form a thin gel layer. Cell solution is added on top of the Matrigel coating to simulate invasion through the extracellular matrix.
Note: There are distinct differences between the transwell cell migration and the transwell cell invasion assays. The transwell cell migration assay measures the chemotactic capability of cells toward a chemo-attractant. The transwell cell invasion assay, however, measures both cell chemotaxis and the invasion of cells through extracellular matrix, a process that is commonly found in cancer metastasis or embryonic development. - Using a pipette, very carefully add 600 μl of the desired chemo-attractant into the bottom of the lower chamber in a 24-well plate. Add the chemo-attractant without moving the transwell insert and avoid generating bubbles. Make sure the chemo-attractant liquid in the bottom well makes contact with the membrane in the upper well to form a chemotactic gradient. Incubation time is dependent on cell type and the chemo-attractant being used.
Note: Further tests may be needed to determine the incubation period.
Note: For adherent cells, the migrated cells will attach to the other side of the membrane1,8. The quantification of migrated cells can be performed following steps 2.4 to 2.8 (steps 2.4-2.8 do not need to be performed in a sterile environment). For non-adherent cells, the migrated cells will drop into the media in the lower chamber. The number of migrated cells can be counted by using hemocytometer or flow cytometer5. - Remove the transwell insert from the plate. Use a cotton-tipped applicator as many times as needed to carefully remove the media and remaining cells that have not migrated from the top of the membrane without damaging it.
- Add 600-1,000 μl of 70% ethanol into a well of a 24-well plate. Place the transwell insert into the 70% ethanol for 10 minutes to allow cell fixation. Remove transwell insert from the 24-well plate and use a cotton-tipped applicator to remove the remaining ethanol from the top of the membrane. Allow the transwell membrane to dry (typically 10-15 minutes).
- Add 600-1,000 μl of 0.2% crystal violet into a well of a 24-well plate and position the membrane into it for staining. Incubate at room temperature for 5-10 minutes.
- Gently remove the crystal violet from the top of the membrane with a pipette tip or cotton tipped applicator. Very carefully, to avoid washing off fixed cells, dip the membrane into distilled water as many times as needed to remove the excess crystal violet. Allow the transwell membrane to dry.
- View underneath an inverted microscope and count the number of cells in different fields of view to get an average sum of cells that have migrated through the membrane toward the chemo-attractant and attached on the underside of the membrane (Figure 3).
Representative Results
The wound closure assay and the transwell cell migration assay presented here were performed using mouse B16F10 melanoma cells as a model system. In the wound closure assay, B16F10 cells were seeded in a 6-well tissue culture plate and grown to 100% confluence in 24 hours. A wound of approximately 700 µm wide was generated using a pipette tip and the wound closure (cell migration) was recorded using the time-lapse microscopy. Alternatively, wound closure can also be studied by taking snapshot pictures at different time points if time-lapse microscopy is not available1. Four pictures from the time-lapse series at 0, 4, 8, and 12-hour time points are shown in Figure 1. Based on the width of the wound, we calculated the migration distance and the speed of the cell migration at 40.42 µm/hr (Figure 1B). The time-lapse pictures were also assembled into a video using the ImageJ program (see supplementary video). The dynamic cell migration process could be studied. As shown in Figure 2, the morphology of migrating cells with lamellipodia (leading edge) and tails (trailing edge) were clearly observed.
In the transwell cell migration assay, 24-well inserts from Corning were used. B16F10 melanoma cells were re-suspended at the concentration of 1 x 106 cells/ml in the migration buffer consisting of DMEM medium and 0.1% BSA without serum. Conditioned media from NIH3T3 fibroblast cells grown in DMEM medium containing 10% fetal bovine serum was used as the chemo-attractant. 100 µl of B16F10 cells was added on top of the transwell membrane in the upper chamber and 600 µl of chemo-attractant was added to the lower chamber or the same volume of migration buffer added as a negative control. Cell migration was performed as described in the Procedure. The number of migrated cells could be quantified by counting underneath a microscope or in the pictures taken (Figure 3B). As shown in Figure 3, there was a 15-fold increase in the cells migrating toward the chemo-attractant in comparison to the control migration buffer (Figure 3C). The transwell assay examines cell chemotaxis, the directional cell migration toward chemo-attractant.

Figure 1. B16F10 melanoma cell wound closure assay. (A) During a 16-hour wound closure assay pictures were taken every 5 minutes using a time-lapse microscope. Representative pictures at 0, 4, 8, and 12 hr are shown and scale bars (400 µm) are added for wound width measurement. (B) The distance of the wound was measured in μm. A scatter plot was used to display the width of the wound over time and the rate of wound closure was calculated. To generate the r2 value, a linear regression was run on the wound width data using the GraphPad Prism software (version 5.0, GraphPad Software, Inc.). Please click here to view a larger version of this figure.

Figure 2. Morphology of migrating B16F10 cells from the time-lapse microscopy of the wound closure assay. During a 16-hour wound closure assay pictures were taken every 5 minutes. All pictures were assembled into a video using the ImageJ program (5 frames/second). Cell migration involving cell polarization, lamellipodia extension, and trailing edge retraction was observed. Pictures from 34.6, 36.6, & 38.6-second time points of the video were presented (video time corresponding to the actual time-lapse migration time (hr:min:sec) 34.6 seconds, 14:20:00; 36.6 seconds, 15:10:00; and 38.6 seconds, 16:00:00, respectively). Scale bar 50 µm. Please click here to view a larger version of this figure.
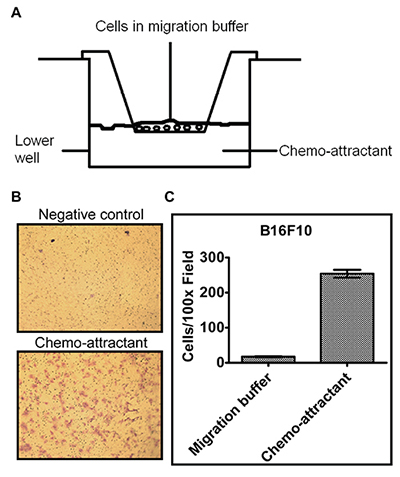
Figure 3. Transwell migration assay of B16F10 melanoma cells. (A) A diagram of the transwell insert apparatus used to measure cell migration and invasion. (B) Representative pictures of B16F10 cell transwell migration. Cell migration buffer and NIH3T3 cell conditioned medium were added to the lower chamber as the negative control and chemo-attractant, respectively. After cell migration and staining with crystal violet as described in the Procedure, pictures of the migrated cells (purple stained) were taken using a microscope with a 10x objective (total magnification 100x). Pores of the membranes could also be observed as the numerous small, round and dark colored dots in the picture. (C) Quantification of cells migrating toward the migration buffer or chemo-attractant(Average of 5 picture fields at 100x total magnification). Please click here to view a larger version of this figure.
Supplementary Video. Time-lapse video of a B16F10 melanoma cell wound closure assay. Pictures were taken every 5 minutes for 16 hours to generate 193 pictures. All pictures were assembled into a video using the ImageJ program (5 frames/second). See the "Supplementary_Video_JOVE.avi" supplementary file under Downloads.
Discussion
Cell migration is an important aspect to study in cancer research and it can also be applied to developmental, immunological and wound healing studies. The cell culture wound closure assay and the transwell cell migration and invasion assays reveal detailed information of cell migratory behaviors and can be used to investigate the molecular mechanisms of cell migration1,2,10,14. Our study used these cell motility assays to determine the migration velocity and invasion capabilities of a B16F10 melanoma cell line.
The cell wound closure assay examines the ability of a particular cell line to migrate and subsequently close a wound made in a confluent plate of cells. This assay is highly accessible to groups with basic equipment and in comparison to existing methods is a simple way to measure cell migration. Some variation in the wound closure assay may be found in individual treatment groups but there are several steps that may be taken to help lessen it. One potential method to reduce variation is to plate each sample with the same number of cells to achieve synchronous confluence and maintain healthy cell status at the initiation of each experiment. Several trials of cell culture experiments may be needed to determine the exact plating number that is needed to achieve invariable confluence and healthy cell status among independent samples. Furthermore, increasing the sample size will also reduce variation. For slow migrating cell lines that require over 24 hour incubation time to observe significant migration, it is convenient to use aphidicolin or other proliferation inhibitor to prevent cell number changes that could potentially affect the outcome of the assay.
After mastering the ability to study cell migration velocity using the cell wound closure assay, a detailed evaluation of single cell migratory behaviors may also be completed14. Furthermore, following a wound closure assay cells may be fixed and various proteins involved with cytoskeletal structure and dynamics may be viewed and evaluated using immunocytochemistry2. This analysis may lead to further detailed biochemical alterations previously unknown. The cell wound closure assay is also highly amenable to real-time live cell imaging to study migration dynamics by using time-lapse fluorescence microscopy. For example, actin-GFP, tubulin-GFP, and paxillin-GFP fusion proteins may be expressed in live cells to view the localization of the proteins at different time points in cell migration or adhesion18,19. Some limitations of the wound closure assay include that, for instance, it is not suitable for non-adherent cells and does not measure cell chemotaxis. In addition, some cell lines have a tendency to detach from the plate immediately after the wound is made (e.g. HEK293T cells). For those cell lines, it is better to use pre-casted wound plates or the transwell migration assay discussed below.
The transwell cell migration and invasion assay provides thorough analysis of the ability of cells to sense a particular chemo-attractant and migrate through a physical barrier toward it. This test can be further used to investigate cell invasion by adding a layer of extracellular matrix or a layer of endothelial cells on top of the transwell membrane to mimic the process of ECM invasion and extravasation1,10. Additionally, following the invasion through Matrigel, immunological staining of cytoskeletal proteins in combination with fluorescence microscopy may be valuable for morphological study during 3-D invasion. A limitation of using the transwell cell invasion assay is that time-lapse data of cell invasion is difficult to attain with conventional microscopy and live cell imaging of this process is complex.
To obtain accurate results, there are several critical steps during the transwell cell migration and invasion assays that are of importance. First, since cell migration velocity may vary widely between different cell types several preliminary experiments must be performed to adopt a specific migration time frame that will be used in the procedure. Second, the chemo-attractant must also be applicable to the cell type of interest. A wide range of chemo-attractants should be examined in preceding experiments before measuring the final migration and invasion capability of a particular cell type. Fibroblasts-conditioned medium is commonly used as a strong chemo-attractant for a wide range of cell types. If a single purified chemo-attractant is used make sure that the receptors of the chemo-attractant are expressed in the cell type of interest. Finally, for the cell invasion assay make sure the coating of Matrigel or other extracellular matrix is homogeneous in order to minimize experimental variation. In conclusion, although there are some limitations, the highly accessible cell migration assays described here are useful for a wide range of biological studies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Mike Myles, Sam Saunders, and C.W. Elton at the ECU Multimedia & Technology Services for providing assistance in video production. We acknowledge the grant support from North Carolina Biotechnology Center, Golfers against Cancer, Brody Brothers Endowment Fund, American Heart Association, and ECU/Vidant Cancer Research and Education Fund (L.V.Y. and M.J.R.).
Materials
| Dulbecco’s modified Eagle medium (DMEM) | Gibco | 11995-073 | |
| Fetal bovine serum (FBS) | Gemini | 100106 | |
| Bovine serum albumin (BSA) | Sigma-Aldrich | A4503-50G | |
| Trypsin EDTA 0.25% | Gibco | 25200-056 | |
| Dulbecco’s phosphate buffered saline (DPBS) | Gibco | 14190-250 | |
| Crystal violet | Sigma | C0775-100G | Dissolved in water at 0.2% |
| Cell disassociation buffer | Gibco | 13151-014 | |
| Cell culture incubator | Thermo Fisher Scientific | Model # 3145 | |
| SterilGARD biosafety hood | The Baker Company, Inc. | Model # VBM-600 | |
| EVOS Fl inverted microscope | Thermo Fisher Scientific | Model # AMF-4302-US | |
| Tissue culture plate | Becton Dickinson | 353046 | Catalog number varies depending on the type of culture plate |
| Corning Transwell insert | Fisher | 07-200-150 | Catalog number varies depending on the pore size of the membrane |
References
- Castellone, R. D., Leffler, N. R., Dong, L., Yang, L. V. Inhibition of tumor cell migration and metastasis by the proton-sensing GPR4 receptor. Cancer Lett. 312 (2), 197-208 (2011).
- Hall, A. The cytoskeleton and cancer. Cancer Metastasis Rev. 28, (2009).
- Lauffenburger, D. A., Horwitz, A. F. Cell migration: a physically integrated molecular process. Cell. 84 (3), 359-369 (1996).
- Peter, C., et al. Migration to apoptotic "find-me" signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 283 (9), 5296-5305 (2008).
- Radu, C. G., Yang, L. V., Riedinger, M., Au, M., Witte, O. N. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc. Natl. Acad. Sci. U. S. A. 101 (1), 245-250 (2004).
- Buul, J. D., Hordijk, P. L. Signaling in leukocyte transendothelial migration. Arterioscler Thromb. Vasc. Biol. (5), 824-833 (2004).
- Yang, L. V., Heng, H. H., Wan, J., Southwood, C. M., Gow, A., Li, L. Alternative promoters and polyadenylation regulate tissue-specific expression of Hemogen isoforms during hematopoiesis and spermatogenesis. 228 (4), 606-616 (2003).
- Yang, L. V., Radu, C. G., Wang, L., Riedinger, M., Witte, O. N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 105 (3), 1127-1134 (2005).
- Yoshida, M., Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 17 (8), 457-465 (2011).
- Albini, A. Tumor and endothelial cell invasion of basement membranes. The matrigel chemoinvasion assay as a tool for dissecting molecular mechanisms. Pathol. Oncol. Res. 4 (3), 230-241 (1998).
- Fidler, I. J. Critical determinants of metastasis. Semin. Cancer Biol. 12 (2), 89-96 (2002).
- Steeg, P. S. Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 12 (8), 895-904 (2006).
- Roussos, E. T., Condeelis, J. S., Patsialou, A. Chemotaxis in cancer. Nat. Rev. Cancer. 11 (8), 573-587 (2011).
- Zhang, Y., et al. Comparative study of 3D morphology and functions on genetically engineered mouse melanoma cells. Integr. Biol. (Camb. 4 (11), 1428-1436 (2012).
- Junger, W. G. Purinergic regulation of neutrophil chemotaxis. Cell. Mol. Life Sci. 65 (16), 2528-2540 (2008).
- Lazennec, G., Richmond, A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 16 (3), 133-144 (2010).
- Huang, H. L., et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 17, (2010).
- Cortesio, C. L., Boateng, L. R., Piazza, T. M., Bennin, D. A., Huttenlocher, A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J. Biol. Chem. 286 (12), 9998-10006 (2011).
- Wehrle-Haller, B., Imhof, B. A. Actin microtubules and focal adhesion dynamics during cell migration. Int. J. Biochem. Cell Biol. 35 (1), 39-50 (2003).

