显微镜和染色:革兰克、胶囊和内孔染色
English
Share
Overview
资料来源:Rhiannon M. LeVeque1, 纳塔利娅·马丁1,安德鲁·范阿尔斯特1,和维克托·迪丽塔1
1密歇根州立大学微生物学和分子遗传学系,美国密歇根州东兰辛分校
细菌是地球上几乎随处可见的各种微生物。许多特性有助于区分它们彼此,包括但不限于革兰染色类型、形状和排列、胶囊的产生和孢子的形成。为了观察这些特性,可以使用光显微镜;然而,一些细菌特性(例如尺寸、缺乏着色和折射特性)使得仅用光学显微镜(1,2)很难区分细菌。使用光显微镜区分细菌类型时,必须染色细菌。光学显微镜的两种主要类型是简单和复合的。它们之间的主要区别是用于放大物体的透镜数量。简单的显微镜(例如放大镜)只有一个镜头来放大物体,而复合显微镜有几个透镜来增强放大倍率(图1)。复合显微镜有一个接近物体的物镜,它收集光来创建物体的图像。然后,通过放大图像的目镜(目镜)放大。与单独使用单个镜头相比,将物镜和目镜相结合可实现更高的放大倍率。通常,复合显微镜具有多个不同功率的物镜,允许不同的放大倍率(1,2)。在这里,我们将讨论可视化细菌与革兰氏污渍,胶囊污渍,和内孔污渍。
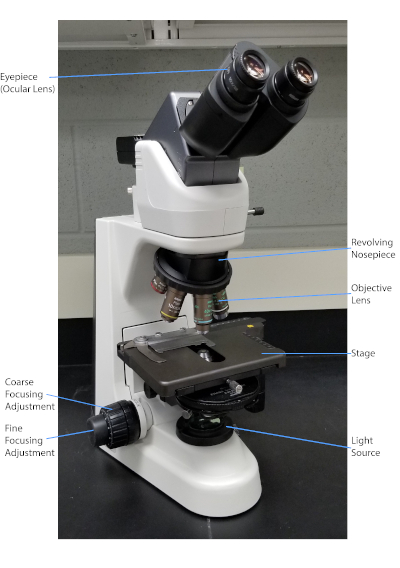
图1:典型的复合显微镜。显微镜最重要的部分是标记的。
1884年由丹麦细菌学家汉斯·克里斯蒂安·格拉姆(1)开发的革兰氏染色剂根据细胞壁的组成(1,2,3,4)区分细菌。简单地说,细菌涂片被放置在显微镜幻灯片上,然后热固定,使细胞粘附在幻灯片上,使它们更容易接受污渍 (1)。热固定样品被水晶紫罗兰染色,使细胞变紫色。幻灯片用碘溶液冲洗,将水晶紫罗兰固定在细胞壁上,然后用去色剂(酒精)洗去任何非固定的水晶紫罗兰。在最后一步中,反伤,萨夫兰宁,被添加到彩色单元格红色(图2)。革兰氏阳性细菌由于厚厚的肽类层而染上紫色,而脱色剂不易渗透;革兰氏阴性细菌,其较薄的肽脂层和较高的脂质含量,与脱色剂脱色,并在添加Safranin时反染红色(图3)。革染用于将细胞区分为两种类型(革兰氏阳性和革兰氏阴性),还可用于区分细胞形状(球体或球菌、棒、弯曲棒和螺旋)和排列(单个细胞、对、链、组和簇)(1、3).
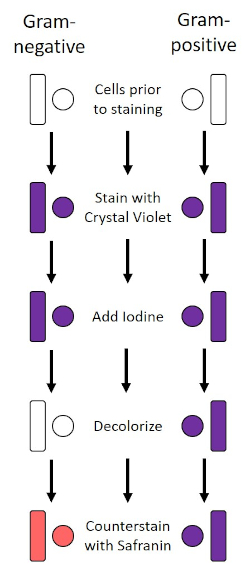
图2:革兰染色协议的原理图。左列显示革兰氏阴性细菌在协议的每一步的反应。右列显示革兰氏阳性细菌的反应。此外,所示是两个典型的细菌细胞形状:杆菌(或棒)和球菌(或球体)。
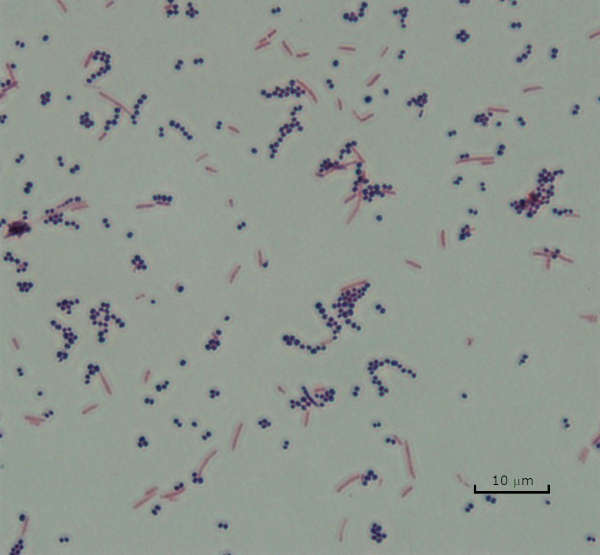
图 3:革兰克染色结果。金黄色葡萄球菌(革联-正紫色球菌)和大肠杆菌(革兰-阴性红棒)混合物的革兰氏染色。
有些细菌产生一个细胞外粘性外层,称为胶囊(3,5)。胶囊是具有各种功能的保护结构,包括但不限于粘附表面和其他细菌、防止干燥和防止噬菌体。胶囊通常由含有95%以上水的多糖组成,但有些可能含有多醇和多胺(5)。由于其大多非离子成分和排斥污渍的倾向,简单的染色方法不适用于胶囊;相反,胶囊染色使用负染色技术,污渍细胞和背景,留下胶囊作为一个明确的光环周围的细胞(1,3)(图4)。胶囊染色涉及将细菌样品涂抹在显微镜幻灯片上的酸性污渍中。与革兰染色不同,细菌污迹在胶囊染色期间不热固定。热固定可以破坏或脱水胶囊,导致假底片 (5)。此外,热固定可以收缩细胞,导致细胞周围的清除,可以误认为胶囊,导致误报(3)。酸性污渍使滑动背景变色;而跟进一个基本的染色,水晶紫罗兰,颜色的细菌细胞本身,离开胶囊不染色,并显示为细胞和滑动背景之间的一个明确的光环(图5)。传统上,印度墨水被用作酸性污渍,因为这些颗粒不能穿透胶囊。因此,胶囊和细胞都没有被印度墨水弄脏;相反,背景被染色。刚果红,尼格罗辛,或Eosin可用于代替印度墨水。胶囊染色可以帮助医生诊断细菌感染时,从患者样本看培养物,并指导适当的患者治疗。由封装细菌引起的常见疾病包括肺炎、脑膜炎和沙门氏菌病。
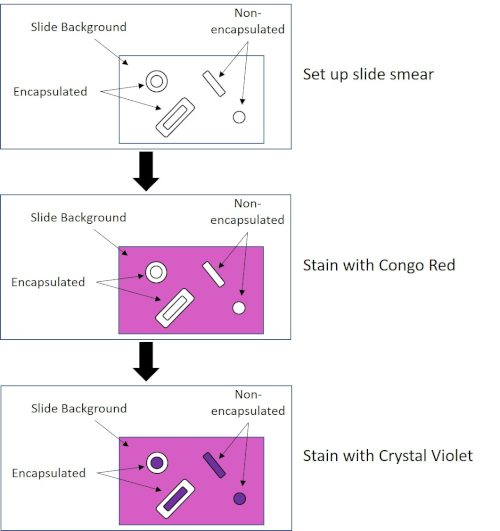
图4:胶囊染色协议原理图。顶部面板显示任何污渍应用前的幻灯片涂抹。中间面板显示幻灯片和细菌如何照顾主要污渍,刚果红。最后一个面板显示幻灯片和细菌如何照顾反污渍,水晶紫罗兰。
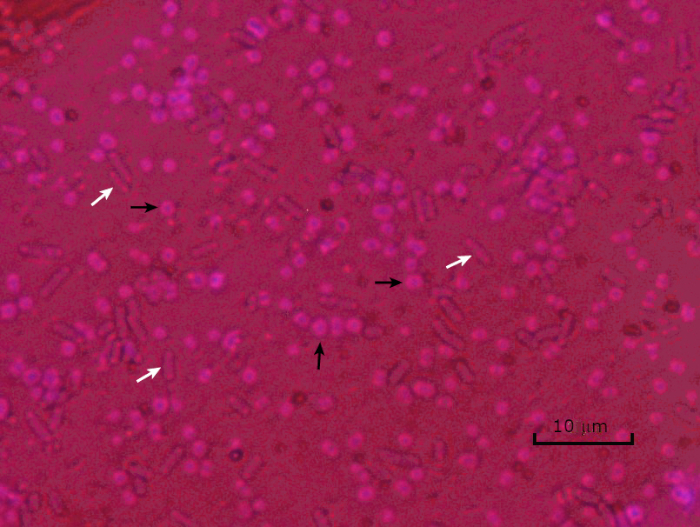
图5:胶囊染色结果。胶囊染色的封装的阿辛托细菌鲍曼尼(用黑色箭头表示)和非封装大肠杆菌(用白色箭头表示)。请注意,背景是黑暗的,A.鲍曼尼细胞是彩色紫色的。A. baumanni细胞周围的胶囊明显是光晕,而大肠杆菌没有光晕。
在不利条件下(例如营养限制、极端温度或脱水),一些细菌产生内孢子,代谢不活性结构,对物理和化学损伤具有抵抗力(1、2、8、9)。内孢子通过保护细胞的遗传物质,使细菌在恶劣的条件下存活;一旦条件有利于生长,孢子发芽,细菌生长继续。内孢子很难用标准染色技术染色,因为它们对通常用于染色的染料是不可渗透的(1,9)。通常用来染色内皮孢子的技术是舍弗-富尔顿法(图6),它使用主要染色马拉奇特绿,水溶性污渍,结合相对较弱的细胞物质和热量,使污渍打破通过孢子皮层(图7)。这些步骤为生长的细胞(在内窥镜生物学中称为植物细胞)以及内孢子和任何游取孢子(那些不再在前细胞包络中的孢子)着色。植物细胞用水洗涤,去除马拉奇特绿;内孢子保留污渍,由于加热马拉奇特绿色在孢子内。最后,植物细胞与萨夫兰宁进行反染色以进行可视化(图8)。内孢子的染色有助于将细菌分化为孢子前体和非孢子前体,并确定样品中是否存在孢子,如果存在,则可能导致细菌在发芽时受到污染。
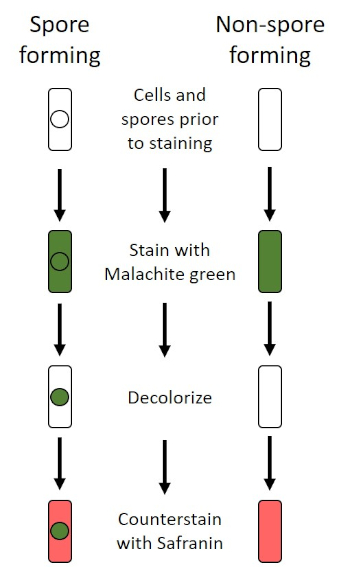
图6:内孔染色协议原理图。左列显示孢子形成细菌在协议的每一步的反应。右列显示非孢子形成细菌的反应。
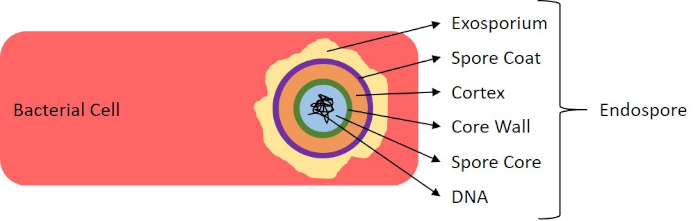
图7:内孔结构图。含有内分孔的细菌细胞,标有各种孢子结构。
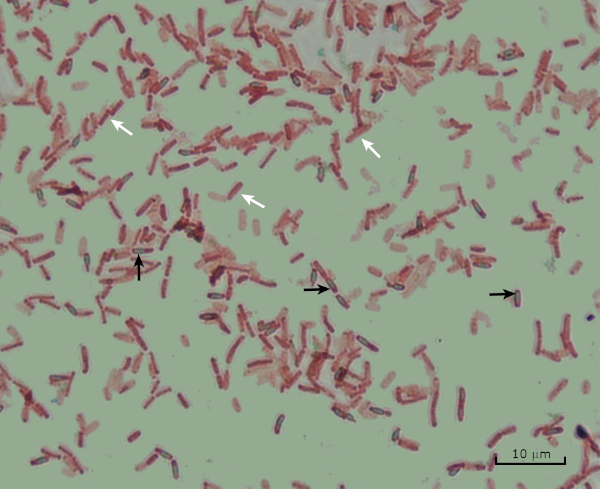
图8:内孔染色结果。亚氏杆菌内皮孢子的典型染色。植物细胞(用白色箭头表示)被染成红色,而内孢子(用黑色箭头表示)被染成绿色。
Procedure
Applications and Summary
Bacteria have distinguishing characteristics that can aid in their identification. Some of these characteristics can be observed by staining and light microscopy. Three staining techniques useful for observing these characteristics are Gram staining, Capsule staining, and Endospore staining. Each technique identifies different characteristics of bacteria and can be used to help physicians recommend treatments for patients, identify potential contaminants in samples or food products, and verify sample sterility.
References
- Black, J. G. Microbiology Principles and Explorations, 4th edition. Prentice-Hall, Inc., Upper Saddle River, New Jersey. (1999)
- Madigan, M. T. and J. M. Martinko. Brock Biology of Microorganisms, 11th edition. Pearson Prentice Hall, Upper Saddle River, New Jersey. (2006).
- Leboffe, M. J., and B. E. Pierce. A Photographic Atlas for the Microbiology Laboratory, 2nd ed. Morton Publishing Company, Englewood, Colorado. (1996).
- Smith, A. C. and M. A. Hussey. Gram stain protocols. Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.2886. (2005).
- Hughes, R. B. and A. C. Smith. Capsule Stain Protocols Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.3041. (2007).
- Anthony, E. E. Jr. A note on capsule staining. Science 73(1890):319-320 (1931).
- Finegold, S. M., W. J. Martin, and E. G. Scott. Bailey and Scott's Diagnostic Microbiology, 5th edition. The C. V. Mosby Company, St. Louis, Missouri. (1978).
- Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg. Methods for general and molecular bacteriology. ASM Press, Washington, DC. (1994).
- Hussey, M. A. and A. Zayaitz. Endospore Stain Protocol. Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.3112. (2007).
Transcript
Bacteria are microscopic living organisms that have many distinguishing characteristics such as shape, arrangement of cells, whether or not they produce capsules, and if they form spores. These features can all be visualized by staining and aid in the identification and classification of different bacterial species.
To examine the first two characteristics of cell shape and arrangement, we can use a simple technique called Gram staining. Here, crystal violet is applied to bacteria, which have been heat-fixed onto a slide. Next, Gram’s iodine solution is added to the slide, resulting in the formation of an insoluble complex between the crystal violet and the Gram’s iodine solution. A decolorizer is then applied and any bacteria with a thick peptidoglycan layer will stain purple, as this layer is not easily penetrated by the decolorizer. These bacteria are referred to as Gram-positive.
Gram-negative bacteria have a thinner peptidoglycan layer and will de-stain the decolorizer, losing the purple color. However, they will stain reddish-pink when a safranin counterstain is added, which binds to a lipopolysaccharide layer on their outside. Once stained, the cells can be observed for morphology, size, and arrangement, such as in chains or clusters, which further aids in classification and identification.
Another useful technique in the microbiologist’s toolkit is the capsule stain, used to visualize external capsules that surround some types of bacterial cells. Due to the capsule’s non-ionic composition and tendency to repel stains, simple staining methods won’t work. Instead, a negative staining technique is used, which first stains the background with an acidic colorant, such as Congo red, before the bacterial cells are stained with crystal violet. This leaves any capsule present as a clear halo around the cells.
The final major staining technique covered here can help determine if the bacteria being studied forms spores. In adverse conditions, some bacteria produce endospores, dormant, tough, non-reproductive structures whose primary function is to ensure the survival of bacteria through periods of environmental stress, like extreme temperatures or dehydration. However, not all bacterial species make endospores, and they are difficult to stain with standard techniques because they are impermeable to many dyes. The Schaeffer-Fulton method uses malachite green stain, which is applied to the bacteria fixed to a slide. The slide is then washed with water before being counterstained with Safranin. Vegetative cells will appear pinkish-red, while any endospores present will appear green. In this video, you will learn how to perform these common bacterial staining techniques and then examine the staining samples using light microscopy.
To begin the procedure, tie back long hair and put on the appropriate personal protective equipment, including a lab coat and gloves.
Then, clean a fresh microscope slide with a laboratory wipe. Next, pipette 10 microliters of 1X phosphate-buffered saline onto the first slide. Then, use a sterile pipette tip to select a single bacterial colony from the LB agar plate. Smear the bacterial colony in the liquid to produce a thin, even layer. Set the slide on the benchtop, and allow it to fully air dry.
Once dried, light a Bunsen burner to heat-fix the bacteria. Using tongs, pass the slide through the burner flame several times, with the bacteria side up, taking care not to hold the slide in the flame too long, which may distort the cells.
Now, working over the sink, hold the slide level and apply several drops of Gram’s crystal violet to completely cover the bacterial smear and then place the slide onto the bench to stand for 45 seconds. Next, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking care not to squirt the bacterial smear directly. Now, holding the slide level again, apply Gram’s iodine solution to completely cover the stained bacteria and then allow it to stand for another 45 seconds. Next, carefully rinse the iodine from the slide, as shown previously. While holding the slide at an angle, add a few drops of Gram’s decolorizer to the slide, allowing it to run down over the stained bacteria, just until the run-off is clear, for approximately 5 seconds. Immediately, rinse with water as shown previously. This will limit over-decolorizing the smear. Next, holding the slide level again, apply Gram’s safranin counterstain to completely cover the stained bacteria. After 45 seconds, gently rinse the Safranin from the slide with water, as shown previously, and then blot dry with paper towels.
Finally, add a drop of immersion oil directly to the slide, and then examine the slide using a light microscope with a 100X oil objective lens.
To begin this staining protocol, first put on the correct personal protective equipment and then ensure that the glass slides that will be used are clean.
Next, prepare the solutions. To make 1% crystal violet solution, mix 0.25 grams of crystal violet powder with 25 milliliters of distilled water and vortex until dissolved. Then, prepare 1% Congo red solution by mixing 0.25 grams of Congo red powder with 25 milliliters of distilled water and vortex until dissolved. Now, pipette 10 microliters of the Congo red solution onto the slide. Using a clean, sterile pipette tip, select a single bacterial colony from the LB agar plate. Then, smear the bacterial colony into the dye to produce a thin, even layer. Completely air dry the bacterial slide for 5-7 minutes. Once the slide is dry, flood the smear with enough 1% crystal violet to cover the smear and let it sit for 1 minute. Now, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking care not to squirt the bacteria directly. Continue holding the slide at a 45-degree angle until completely air-dried. Finally, add a drop of immersion oil directly to the slide, and then examine the slide using a light microscope with a 100X oil objective.
To perform endospore staining, first, prepare a 0.5% malachite green solution by mixing 0. 125 grams of malachite green powder with 25 milliliters of distilled water, and then vortex the solution until dissolved. Next, pipette 10 microliters of 1X PBS onto the center of the slide. Then, use a sterile pipette tip to select a single bacterial colony from the LB agar plate. Smear the bacteria into the liquid to produce a thin, even layer. Now, set the slide on the benchtop, and allow it to fully air dry. Once dried, light a Bunsen burner to heat-fix the bacteria. Pass the slide through the blue burner flame several times, with the bacteria side facing up. Then, once the slide has cooled, place a piece of precut lens paper over the heat-fixed smear. Next, turn on a hotplate to the highest setting, and bring a beaker of water to a boil.
Saturate the lens paper with the malachite green solution and, using tongs, place the slide on top of the beaker of boiling water to steam for 5 minutes. Keep the lens paper moist by adding more dye, one drop at a time, as needed. Next, again using tongs, pick up the slide from the beaker and remove and discard the lens paper. Allow the slide to cool for 2 minutes. Working over the sink, hold the slide at an angle, and gently squirt a stream of water onto the top of the slide. Now, hold the slide level and apply Safranin to completely cover the slide. Then, allow it to stand for 1 minute. Next, hold the slide at an angle and rinse as previously shown. Allow the slide to air dry on the benchtop. Finally, add a drop of immersion oil directly to the slide, and then examine the slide with a light microscope, with a 100X oil objective.
In the Gram staining protocol, two different colored stains can result. Dark purple staining indicates that the bacteria are Gram-positive and that they have retained the crystal violet stain. In contrast, reddish-pink staining is a characteristic of Gram-negative bacteria, which instead will be colored by the Safranin counterstain. Additionally, different shapes and arrangements of bacteria can be visualized after Gram staining. For example, it is possible to differentiate Cocci, or round bacteria, from rod-shaped Bacillus, or identify bacteria, which forms strands, compared to those which typically aggregate as clumps or occur singly.
In a capsule stained microscope image, the bacterial cells will typically be stained purple, and the background of the slide should be darkly stained. Against this dark background, the capsules of the bacteria, if present, will appear as a clear halo around the cells.
Lastly, in endospore staining, Vegetative cells will be stained red by the Safranin counterstain. If endospores are present in the sample, these will retain the malachite green stain and appear bluish-green in color.
