18.9: Corrosion
The degradation of metals due to natural electrochemical processes is known as corrosion. Rust formation on iron, tarnishing of silver, and the blue-green patina that develops on copper are examples of corrosion. Corrosion involves the oxidation of metals. Sometimes it is protective, such as the oxidation of copper or aluminum, wherein a protective layer of metal oxide or its derivatives forms on the surface, protecting the underlying metal from further oxidation. In other cases, corrosion is damaging to the metal, such as the rusting of iron.
Undesirable Redox Reaction: The Rusting of Iron
Rusting occurs due to exposure of iron to oxygen and water. Rust formation involves the creation of a galvanic cell at the surface of iron, which results in the generation of iron(II). The relevant redox reactions that occur at the anodic (oxidation of iron) and cathodic (reduction of oxygen) regions formed on the iron surface include:
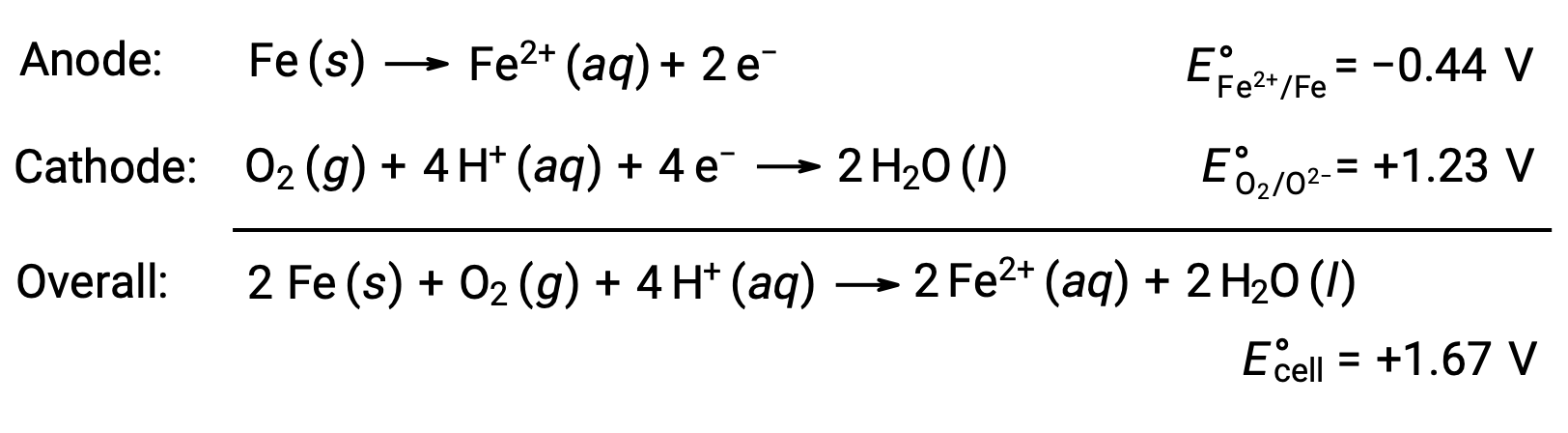
The iron(II) further reacts with humid air, forming an iron(III) oxide hydrate, commonly known as rust.
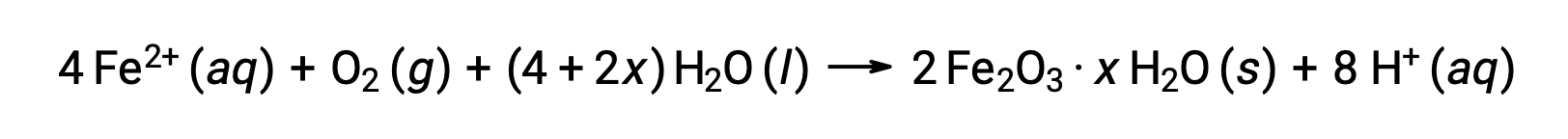
The hydrate's stoichiometry varies with the amount of water to which iron(II) is exposed, as indicated by the use of x in the compound formula. Moisture, the presence of acids and electrolytes increases the rate of formation of rust. Unlike the patina on copper, the formation of rust does not create a protective layer, and so corrosion of the iron continues as the rust flakes off and exposes fresh iron to the atmosphere.
Prevention of Corrosion
Various methods can be used to prevent corrosion. One way is to keep the metal surface painted to avoid contact with water and oxygen. Alloying of metals, like mixing iron with small amounts of chromium in stainless steel, is another effective method to prevent corrosion. The chromium collects near the surface and undergoes oxidation, thus, effectively protecting the iron from corrosion.
Iron and other metals may also be protected from corrosion by galvanization, a process in which the metal to be protected is coated with a layer of a more readily oxidized metal, usually zinc. When the zinc layer is intact, it prevents air from contacting the underlying iron and thus prevents corrosion. If the zinc layer is breached by either corrosion or mechanical abrasion, the iron may still be protected from corrosion by a cathodic protection process, which is described in the next paragraph.
Cathodic protection utilizes the principle of converting the metal to be protected into a cathode in an electrochemical reaction. This is achieved by connecting the protected metal to a more active or easily oxidized metal such as zinc or magnesium, known as the sacrificial anode. The anode corrodes and gets used up protecting the metal that serves as the cathode. Cathodic protection is most commonly used in household appliances such as water heaters and underground water storage tanks. Importantly, cathodic protection can be used for metals other than just iron.
This text is adapted from OpenStax, Chemistry 2e, Chapter 17.6: Corrosion.


