16.5: 緩衝液の有効性
緩衝液は、pHを一定に保つ能力がずっと維持されるわけではありません。緩衝液がpHの変化に耐えられるかどうかは、共役系の弱酸・弱塩基ペアが十分存在するかどうかにかかっています。十分量の強酸または強塩基によって緩衝ペアのどちらかの濃度が大幅に低下すると、溶液内の緩衝作用が損なわれます。
緩衝能とは、一定量の緩衝液に酸や塩基を加えてもpHが大きく変化しない量のことで、通常は単位ごとに表されます。緩衝能は、緩衝液に含まれる弱酸とその共役塩基の量に依存します。例えば、1.0 Mの酢酸と1.0 Mの酢酸ナトリウムからなる溶液1 Lは、0.10 Mの酢酸と0.10 Mの酢酸ナトリウムからなる溶液1Lよりも、同じpHであっても緩衝能が高いです。前者の溶液の方がより多くの酢酸と酢酸イオンを含んでいるため、より大きな緩衝能を有しています。
適切な緩衝液の選択
緩衝液の混合物を選択する際には、2つの有用な経験則があります。
- 良い緩衝液は、両成分の濃度がほぼ等しいです。緩衝液は、一方の成分が他方の成分の約10%未満である場合、一般的にその緩衝作用を失います。
- pHが7以下の場合は弱酸とその塩、pHが7以上の場合は弱塩基とその塩が緩衝剤として適しています。
血液は緩衝液の重要な例であり、緩衝作用を担う主な酸とイオンは、炭酸(H2CO3)と炭酸水素イオン(HCO3−)です。ヒドロニウムイオンが新たに血中に加わると、主にこの反応によって消費されます。

新たに加わった水酸化物イオンは、次の反応によって消費されます。:
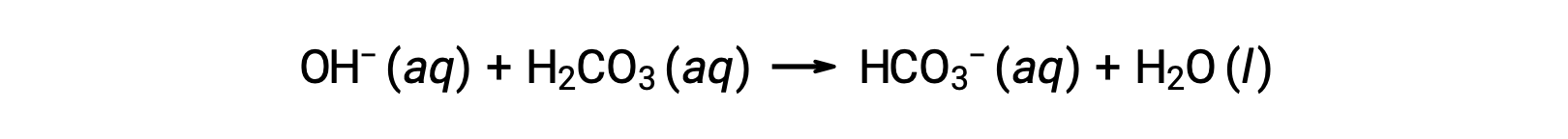
このようにして、添加された強酸または強塩基は、緩衝剤ペアのはるかに弱い酸または塩基に変換されます(H3O+はH2CO3に変換され、OH−はHCO3−に変換される)。このようにして、ヒトの血液のpHは、緩衝剤ペアのpKaによって決定される値、この場合は7.35に非常に近い値を維持しています。血液のpHの正常な変化は通常0.1以下であり、0.4以上のpHの変化は致命的であると考えられています。
上記の文章は以下から引用しました。 Openstax, Chemistry 2e, Section 14.6: Buffers.


